Abstract
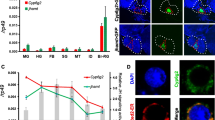
In insects, the follicle cells (FCs) give rise to a single-layered tissue of binucleated professional secretory cells that surround the oocytes during oogenesis. In the latest stage of oocyte development, the FCs rapidly synthesize and secrete the chorion (eggshell) immediately before degenerating through apoptosis. Here, we used RT-qPCR, electron microscopy, and RNAi silencing to explore the role of the main unfolded protein response (UPR) receptors IRE1 and PERK, as well as the ultrastructure dynamics of the FCs during oogenesis of the insect vector of Chagas disease Rhodnius prolixus. We found that IRE1 and PERK mRNAs are highly expressed in the ovaries of vitellogenic females. Interestingly, we observed that IRE1 and PERK, as well as different isoforms of the chaperones Bip and PDI, have their FCs gene expression levels decreased during the vitellogenesis to choriogenesis transition. Using transmission electron microscopy, we observed that the downregulation of the UPR gene expression is accompanied by dramatic changes in the FCs ultrastructure, with an 80% reduction in the mean area of the ER tubules, and circularization and enlargement of the mitochondria. Additionally, we found that parental RNAi silencing of both IRE1 and PERK resulted in minor changes in the chorion protein composition and ultrastructure, accessed by urea extraction of the chorion proteins and scanning electron microscopy, respectively, but did not impact the overall levels of oviposition and F1 embryo development.





Similar content being viewed by others
References
Anderson LM, Telfer WH (1969) A follicle cell contribution to the yolk spheres of moth oocytes. Tissue Cell 1:633–644. https://doi.org/10.1016/s0040-8166(69)80037-6
Bomfim L, Ramos I (2020) Deficiency of ULK1/ATG1 in the follicle cells disturbs ER homeostasis and causes defective chorion deposition in the vector Rhodnius prolixus. FASEB J off Publ Fed Am Soc Exp Biol 34:13561–13572. https://doi.org/10.1096/fj.202001396R
Bouts DMD, do Amaral Melo AC, Andrade ALH et al (2007) Biochemical properties of the major proteins from Rhodnius prolixus eggshell. Insect Biochem Mol Biol 37:1207–1221. https://doi.org/10.1016/j.ibmb.2007.07.010
Bownes M, Nöthiger R (1981) Sex determining genes and vitellogenin synthesis in Drosophila melanogaster. Mol Gen Genet 182:222–228. https://doi.org/10.1007/BF00269661
Brennan MD, Weiner AJ, Goralski TJ, Mahowald AP (1982) The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev Biol 89:225–236. https://doi.org/10.1016/0012-1606(82)90309-8
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
de Bianchi AG, Coutinho M, Pereira SD et al (1985) Vitellogenin and vitellin of Musca domestica quantification and synthesis by fat bodies and ovaries. Insect Biochem 15:77–84. https://doi.org/10.1016/0020-1790(85)90047-2
Deng WM, Bownes M (1998) Patterning and morphogenesis of the follicle cell epithelium during Drosophila oogenesis. Int J Dev Biol 42:541–552. https://doi.org/10.1387/ijdb.9694625
Fausto AM, Fava E, Mazzini M et al (1998) Confocal scanning laser microscopy of the follicular epithelium in ovarioles of the stick insect Carausius morosus. Cell Tissue Res 293:551–561. https://doi.org/10.1007/s004410051147
Fiil A (1978) Follicle cell bridges in the mosquito ovary: syncytia formation and bridge morphology. J Cell Sci 31:137–143
Fourney RM, Pratt GF, Harnish DG et al (1982) Structure and synthesis of vitellogenin and vitellin from Calliphora erythrocephala. Insect Biochem 12:311–321. https://doi.org/10.1016/0020-1790(82)90089-0
Giorgi F (1978) Intercellular bridges in ovarian follicle cells of Drosophila melanogaster. Cell Tissue Res 186:413–422. https://doi.org/10.1007/BF00224931
Gutzeit HO (1990) The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur J Cell Biol 53:349–356
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. https://doi.org/10.1038/nrm3270
Huang HW, Zeng X, Rhim T et al (2017) The requirement of IRE1 and XBP1 in resolving physiological stress during Drosophila development. J Cell Sci 130:3040–3049. https://doi.org/10.1242/jcs.203612
Huebner E, Anderson E (1972a) A cytological study of the ovary of Rhodnius prolixus I. The ontogeny of the follicular epithelium. J Morphol 136:459–493
Huebner E, Anderson E (1972b) A cytological study of the ovary of Rhodnius prolixus Morphol 137:385–415
Huebner E, Anderson E (1972c) A cytological study of the ovary of Rhodnius prolixus. III. Cytoarchitecture and development of the trophic chamber. J Morphol 138:1–39. https://doi.org/10.1002/jmor.1051380102
Huebner E, Tobe SS, Davey KG (1975) Structural and functional dynamics of oogenesis in Glossina austeni: vitellogenesis with special reference to the follicular epithelium. Tissue Cell 7:535–558. https://doi.org/10.1016/0040-8166(75)90025-7
Irie K, Yamashita O (1983) Egg-specific protein in the silkworm Bombyx mori: purification properties localization and titre changes during oogenesis and embryogenesis. Insect Biochem 13:71–80. https://doi.org/10.1016/0020-1790(83)90066-5
Javadov S, Chapa-Dubocq X, Makarov V (2018) Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 38:58–70. https://doi.org/10.1016/j.mito.2017.08.004
Karbowski M, Youle RJ (2003) Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 10:870–880. https://doi.org/10.1038/sj.cdd.4401260
Kimber SJ (1980) The secretion of the eggshell of Schistocerca gregaria: ultrastructure of the follicle cells during the termination of vitellogenesis and eggshell secretion. J Cell Sci 46:455–477
Kunkel JG (1991) Models of pattern formation in insect oocytes. In Vivo (brooklyn) 5:443–456
Medeiros MN, Logullo R, Ramos IB et al (2011) Transcriptome and gene expression profile of ovarian follicle tissue of the triatomine bug Rhodnius prolixus. Insect Biochem Mol Biol 41:823–831. https://doi.org/10.1016/j.ibmb.2011.06.004
Melius ME, Telfer WH (1969) An autoradiographic analysis of yolk deposition in the cortex of the cecropia moth oocyte. J Morphol 129:1–15. https://doi.org/10.1002/jmor.1051290102
Melo AC, Valle D, Machado EA et al (2000) Synthesis of vitellogenin by the follicle cells of Rhodnius prolixus. Insect Biochem Mol Biol 30:549–557
Merril CR, Dunau ML, Goldman D (1981) A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem 110:201–207
Mesquita RD, Vionette-Amaral RJ, Lowenberger C et al (2015) Genome of Rhodnius prolixus an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc Natl Acad Sci 112:14936–14941. https://doi.org/10.1073/pnas.1506226112
Nunes-da-fonseca R, Berni M, Pane A, Araujo HM (2017) Rhodnius prolixus: from classical physiology to modern developmental biology. Genesis 55(5):e22995. https://doi.org/10.1002/dvg.22995
Peferoen M, Loof ADE (1986) Synthesis of vitellogenic and non-vitellogenic yolk proteins by the fat body and the ovary of Leptinotarsa decemlineata. Comp Biochem Physiol Pt B Comp Biochem 83(1):251–254
Pereira J, Diogo C, Fonseca A et al (2020) Silencing of RpATG8 impairs the biogenesis of maternal autophagosomes in vitellogenic oocytes, but does not interrupt follicular atresia in the insect vector Rhodnius prolixus. PLoS Negl Trop Dis 14:e0008012. https://doi.org/10.1371/journal.pntd.0008012
Perri ER, Thomas CJ, Parakh S et al (2016) The unfolded protein response and the role of protein disulfide isomerase in neurodegeneration. Front Cell Dev Biol 3:1–17. https://doi.org/10.3389/fcell.2015.00080
Picard M, Shirihai OS, Gentil BJ, Burelle Y (2013) Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol 304:R393–R406. https://doi.org/10.1152/ajpregu.00584.2012
Pobre KFR, Poet GJ, Hendershot LM (2019) The endoplasmic reticulum (ER) chaperone Bip is a master regulator of ER functions: getting by with a little help from ERdj friends. J Biol Chem 294:2098–2108. https://doi.org/10.1074/jbc.REV118.002804
Ramamurty PS, Engels W (1977) Occurrence of intercellular bridges between follicle epithelial cells in the ovary of Apis mellifica queens. J Cell Sci 24:195–202
Rowland AA, Voeltz GK (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13:607–625. https://doi.org/10.1038/nrm3440
Ryoo HD (2015) Drosophila as a model for unfolded protein response research. BMB Rep 48:445–453. https://doi.org/10.5483/BMBRep.2015.48.8.099
Santos A, Ramos I (2021) ATG3 is important for the chorion ultrastructure during oogenesis in the insect vector Rhodnius prolixus. Front Physiol 12:1–11. https://doi.org/10.3389/fphys.2021.638026
Schwarz DS, Blower MD (2016) The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73:79–94. https://doi.org/10.1007/s00018-015-2052-6
Song S, Tan J, Miao Y, Zhang Q (2018) Crosstalk of ER stress-mediated autophagy and ER-phagy: involvement of UPR and the core autophagy machinery. J Cell Physiol 233:3867–3874. https://doi.org/10.1002/jcp.26137
Souid S, Lepesant JA, Yanicostas C (2007) The xbp-1 gene is essential for development in Drosophila. Dev Genes Evol 217:159–167. https://doi.org/10.1007/s00427-006-0124-1
Stein DS, Stevens LM (2014) Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip Rev Dev Biol 3:301–330. https://doi.org/10.1002/wdev.138
Vieira PH, Bomfim L, Atella GC et al (2018) Silencing of RpATG6 impaired the yolk accumulation and the biogenesis of the yolk organelles in the insect vector R prolixus. PLoS Negl Trop Dis 12:e0006507. https://doi.org/10.1371/journal.pntd.0006507
Vogel E, Santos D, Mingels L et al (2019) RNA interference in insects: protecting beneficials and controlling pests. Front Physiol 10:1–21. https://doi.org/10.3389/fphys.2018.01912
Wang CC (1998) Protein disulfide isomerase assists protein folding as both an isomerase and a chaperone. Ann NY Acad Sci 864:9–13. https://doi.org/10.1111/j.1749-6632.1998.tb10283.x
Wang L, Wang X, Wang C (2015) Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic Biol Med 83:305–313. https://doi.org/10.1016/j.freeradbiomed.2015.02.007
Weng SC, Shiao SH (2020) The unfolded protein response modulates the autophagy-mediated egg production in the mosquito Aedes aegypti. Insect Mol Biol 29:404–416. https://doi.org/10.1111/imb.12645
Westrate LM, Lee JE, Prinz WA, Voeltz GK (2015) Form follows function: the importance of endoplasmic reticulum shape. Annu Rev Biochem 84:791–811. https://doi.org/10.1146/annurev-biochem-072711-163501
WHO (2019a) Chagas disease (also known as American trypanosomiasis). https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
World Health Organization (2019b) Chagas disease (also known as American trypanosomiasis). https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
Wu X, Tanwar PS, Raftery LA (2008) Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol 19:271–282. https://doi.org/10.1016/j.semcdb.2008.01.004
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304. https://doi.org/10.1074/jbc.M607007200
Zhai QH, Postlethwait JH, Bodley JW (1984) Vitellogenin synthesis in the lady beetle Coccinella septempunctata. Insect Biochem 14:299–305. https://doi.org/10.1016/0020-1790(84)90064-7
Zongza V, Dimitriadis GJ (1988) Vitellogenesis in the insect Dacus oleae Isolation and characterization of yolk protein mRNA. Insect Biochem 18:651–660. https://doi.org/10.1016/0020-1790(88)90073-X
Acknowledgements
The authors thank Yasmin Gutierrez and Yan Mendonça for the careful care of our lab insectarium and CENABIO-UFRJ for providing electron microscopy equipment and facilities.
Funding
This work was funded by the following grants: JCNE-FAPERJ (www.faperj.br/), INCT-EM-CNPq/FAPERJ (http://cnpq.br/), and CAPES (www.capes.gov.br/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was funded by the following grants. Fundação Carlos Chagas Filho De Amparo À Pesquisa Do Estado Do Rio De Janeiro (FAPERJ) (JCNE E-26/2031802017; http://www.faperj.br/) to I.R.; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (INCT-EM 16/2014; http://cnpq.br/) to I.R.; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (https://www.gov.br/capes/pt-br).
Author information
Authors and Affiliations
Contributions
T.R. and L.B. designed and conducted the experiments and revised the paper; I.R. designed the experiments and wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
All animal care and experimental protocols were approved by guidelines of the institutional care and use committee (Committee for Evaluation of Animal Use for Research from the Federal University of Rio de Janeiro, CEUA-UFRJ #01200.001568/2013–87, order number 155/13), under the regulation of the national council of animal experimentation control (CONCEA).
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thamara Rios and Larissa Bomfim equally contributed to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rios, T., Bomfim, L. & Ramos, I. The transition from vitellogenesis to choriogenesis triggers the downregulation of the UPR sensors IRE1 and PERK and alterations in the ER architecture in the follicle cells of the vector Rhodnius prolixus. Cell Tissue Res 387, 63–74 (2022). https://doi.org/10.1007/s00441-021-03547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03547-z