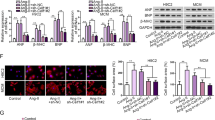
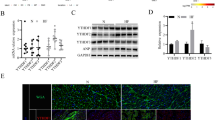
Abstract
Cardiac hypertrophy is considered as a common pathophysiological process in various cardiovascular diseases. CUG triplet repeat-binding protein 1 (CELF1) is an RNA-binding protein that has been shown to be an important post-transcription regulator and involved in several types of cancer, whereas its role in cardiac remodeling remains unclear. Herein, we found that the expression of CELF1 was significantly increased in pressure overload-induced hypertrophic hearts and angiotensin II (Ang II)-induced neonatal cardiomyocytes. Based on transverse aortic constriction-induced cardiac hypertrophy model, CELF1 deficiency markedly ameliorated cardiac hypertrophy, cardiac fibrosis, oxidative stress, and apoptosis. Accordingly, CELF1 deficiency alleviated the production of reactive oxygen species (ROS) and apoptosis of neonatal cardiomyocytes via inhibition of Raf1, TAK1, ERK1/2, and p38 phosphorylation. Mechanistically, depletion or overexpression of CELF1 negatively regulated the protein expression of phosphatidylethanolamine-binding protein 1 (PEBP1), while the mRNA expression of PEBP1 remained unchanged. RNA immunoprecipitation revealed that CELF1 directly interacted with PEBP1 mRNA. Biotin pull-down analysis and dual-luciferase assay showed that CELF1 directly bound to the fragment 1 within 3’UTR of PEBP1. Moreover, knockdown of PEBP1 partially enhanced the production of ROS and apoptosis of neonatal cardiomyocytes inhibited by CELF1 deficiency. In conclusion, CELF1 binds to the 3’UTR of PEBP1 and acts as an endogenous activator of MAPK signaling pathway. Inhibition of CELF1 attenuates pathological cardiac hypertrophy, oxidative stress, and apoptosis, thus could be a potential therapeutic strategy of pathological cardiac hypertrophy.






Similar content being viewed by others
Data availability
The data used to support the findings of this study are included within the article.
References
Blech-Hermoni Y, Dasgupta T, Coram RJ, Ladd AN (2016) Identification of targets of CUG-BP, Elav-like family member 1 (CELF1) regulation in embryonic heart muscle. PLoS One 11:e0149061
Brinegar AE, Cooper TA (2016) Roles for RNA-binding proteins in development and disease. Brain Res 1647:1–8
Chang KT, Cheng CF, King PC, Liu SY, Wang GS (2017) CELF1 mediates connexin 43 mRNA degradation in dilated cardiomyopathy. Circ Res 121:1140–1152
Chang KT, Wang LH, Lin YM, Cheng CF, Wang GS (2021) CELF1 promotes vascular endothelial growth factor degradation resulting in impaired microvasculature in heart failure. FASEB J 35:e21512
Ciolli Mattioli C, Rom A, Franke V, Imami K, Arrey G, Terne M, Woehler A, Akalin A, Ulitsky I, Chekulaeva M (2019) Alternative 3’ UTRs direct localization of functionally diverse protein isoforms in neuronal compartments. Nucleic Acids Res 47:2560–2573
Cox DC, Guan X, Xia Z, Cooper TA (2020) Increased nuclear but not cytoplasmic activities of CELF1 protein leads to muscle wasting. Hum Mol Genet 29:1729–1744
deAlmeida AC, van Oort RJ, Wehrens XH (2010) Transverse aortic constriction in mice. J Vis Exp 38:1729
Giudice J, Xia Z, Li W, Cooper TA (2016) Neonatal cardiac dysfunction and transcriptome changes caused by the absence of Celf1. Sci Rep 6:35550
Hakem Zadeh F, Teng ACT, Kuzmanov U, Chambers PJ, Tupling AR, Gramolini AO (2019) AKAP6 and phospholamban colocalize and interact in HEK-293T cells and primary murine cardiomyocytes. Physiol Rep 7:e14144
Jia W, Bai T, Zeng J, Niu Z, Fan D, Xu X, Luo M, Wang P, Zou Q, Dai X (2021) Combined administration of metformin and atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in type 2 diabetic mice. Front Cell Dev Biol 9:634900
Jones K, Timchenko L, Timchenko NA (2012) The role of CUGBP1 in age-dependent changes of liver functions. Ageing Res Rev 11:442–449
Kalsotra A, Cooper TA (2011) Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet 12:715–729
Kim JH, Kwon HY, Ryu DH, Nam MH, Shim BS, Kim JH, Lee JY, Kim SH (2017) Inhibition of CUG-binding protein 1 and activation of caspases are critically involved in piperazine derivative BK10007S induced apoptosis in hepatocellular carcinoma cells. PLoS One 12:e0186490
Klein AF, Dastidar S, Furling D, Chuah MK (2015) Therapeutic approaches for dominant muscle diseases: highlight on myotonic dystrophy. Curr Gene Ther 15:329–337
Lewis K, Valanejad L, Cast A, Wright M, Wei C, Lakova P, Stock L, Karns R, Timchenko L, Timchenko N (2017) RNA Binding Protein CUGBP1 Inhibits Liver Cancer in a Phosphorylation-Dependent Manner. Mol Cell Biol 37:e00128-17
Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, Davila-Roman VG, Stein RI, Dorn GW 2nd, Gropler RJ et al (2011) Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (silver Spring) 19:1804–1812
Liu Y, Wang H, Wang J, Wei B, Zhang X, Zhang M, Cao D, Dai J, Wang Z, Nyirimigabo E et al (2019) A positive feedback regulation of Heme oxygenase 1 by CELF1 in cardiac myoblast cells. Biochim Biophys Acta Gene Regul Mech 1862:209–218
Mastroleo I (2016) Post-trial obligations in the Declaration of Helsinki 2013: classification, reconstruction and interpretation. Dev World Bioeth 16:80–90
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407
Parameswaran S, Santhakumar R, Vidyasekar P, Verma RS (2015) Enrichment of cardiomyocytes in primary cultures of murine neonatal hearts. Methods Mol Biol 1299:17–25
Pitoulis FG, Nunez-Toldra R, Xiao K, Kit-Anan W, Mitzka S, Jabbour RJ, Harding SE, Perbellini F, Thum T, de Tombe PP, Terracciano CM (2021) Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. Cardiovasc Res. https://doi.org/10.1093/cvr/cvab084
Raquel-Cunha A, Cardoso-Carneiro D, Reis RM, Martinho O (2019) Current Status of Raf Kinase Inhibitor Protein (RKIP) in Lung Cancer: Behind RTK Signaling. Cells 8:442
Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, St Louis-Vlasova IA, Bohjanen PR (2010) Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol 30:3970–3980
Rautaharju PM, Soliman EZ (2014) Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: a critical appraisal. J Electrocardiol 47:649–654
Schmid E, Neef S, Berlin C, Tomasovic A, Kahlert K, Nordbeck P, Deiss K, Denzinger S, Herrmann S, Wettwer E et al (2015) Cardiac RKIP induces a beneficial beta-adrenoceptor-dependent positive inotropy. Nat Med 21:1298–1306
Tanaka K, Honda M, Takabatake T (2001) Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol 37:676–685
Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR (2015) Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol 89:1401–1438
van der Meer P, Gaggin HK, Dec GW (2019) ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J Am Coll Cardiol 73:2756–2768
Wang GS, Kearney DL, De Biasi M, Taffet G, Cooper TA (2007) Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J Clin Invest 117:2802–2811
Wang S, Zhang Y, Cai Q, Ma M, Jin LY, Weng M, Zhou D, Tang Z, Wang JD, Quan Z (2019) Circular RNA FOXP1 promotes tumor progression and Warburg effect in gallbladder cancer by regulating PKLR expression. Mol Cancer 18:145
Xia L, Sun C, Li Q, Feng F, Qiao E, Jiang L, Wu B, Ge M (2015) CELF1 is up-regulated in glioma and promotes glioma cell proliferation by suppression of CDKN1B. Int J Biol Sci 11:1314–1324
Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H et al (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401:173–177
Zhou L, Miao K, Yin B, Li H, Fan J, Zhu Y, Ba H, Zhang Z, Chen F, Wang J et al (2018) Cardioprotective role of myeloid-derived suppressor cells in heart failure. Circulation 138:181–197
Funding
This work was supported by Program of Tianjin Science and Technology Plan (Grant No. 18YFZCSY01080).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaomin Hu and Peng Wu are co-first authors.
Rights and permissions
About this article
Cite this article
Hu, X., Wu, P., Liu, B. et al. RNA-binding protein CELF1 promotes cardiac hypertrophy via interaction with PEBP1 in cardiomyocytes. Cell Tissue Res 387, 111–121 (2022). https://doi.org/10.1007/s00441-021-03541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03541-5