Abstract
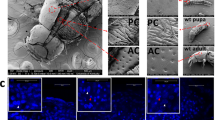
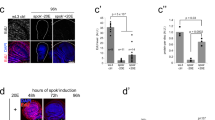
The winter moth, Nyssiodes lefuarius, exhibits striking sexual dimorphism in wing form; males have functional wings of normal size, whereas females lack wings. We previously found that the steroid hormone 20-hydroxyecdysone (20E) triggered massive programmed cell death (PCD) only in the female pupal wing epithelium; however, when and how early sexual trait development of the pupal wings is initiated during pupal-adult metamorphosis remains obscure. To clarify the detailed morphological changes and mechanisms underlying early sexual trait development and cell death, we examined the effects of 20E on early ultrastructural and histological changes in the pupal wing epithelium of both sexes. Before the onset of adult differentiation, no morphological differences were observed in the epithelial cells of both sexes at an ultrastructural level. When 5.4 µg of 20E was injected into pupae of both sexes at 15 days after the onset of pupation, retraction of the wing epithelium from the pupal cuticle was initiated at day 2 after 20E injection in both sexes. Although overt degeneration of wing tissue was not still obvious, apoptotic body-like structures and auto-phagosomes were visible at day 3 after 20E injection in females, whereas development of scale precursor cells started on day 4 after injection in males. Our results suggest that (1) the injection of 20E induced sexually dimorphic changes in the pattern of organelle distribution in wing epithelial cells, and (2) abnormally shaped mitochondria in the cytoplasm of the female wing epithelium might be involved in the PCD that occurs during wing tissue degeneration.







Similar content being viewed by others
References
Bayer EA, Sun H, Rafi I, Hobert O (2020) Temporal, spatial, sexual and environmental regulation of the master regulator of sexual differentiation in C. elegans. Curr Biol 30:3604–3616
Bear A, Monteiro A (2013) Both cell-autonomous mechanisms and hormones contribute to sexual development in vertebrates and insects. BioEssays 35:725–732
Clarke PGH (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213
Dohrmann CF, Nijhout NF (1988) Development of the wing margin in Precis coenia (Lepidoptera: Nymphalidae). J Res Lep 27:151–159
Dyche WJ (1979) A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol 162:175–209
Ellis RE, Horvitz HR (1991) Two C. elegans genes control the programmed cell deaths of specific cells in the pharynx. Development 112:591–603
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147:742–758
Fujiwara H, Ogai S (2001) Ecdysteroid-induced programmed cell death and cell proliferation during pupal wing development of the silkworm, Bombyx mori. Dev Gene Evol 211:188–123
Gempe T, Beye M (2011) Function and sex determination mechanisms, genes, and pathways in insects. BioEssays 33:52–60
Ghose P, Shaham S (2020) Cell death in animal development. Development 147: dev191882. https://doi.org/10.1242/dev.191882
Glücksmann A (1951) Cell deaths in normal vertebrate ontogeny. Biol Rev 26:59–86
Hunter AF (1995) The ecology and evolution of reduced wings in forest macrolepidoptera. Evol Ecol 9:275–287
Ishizuya-Oka A, Hasebe T, Shi Y-B (2010) Apoptosis in amphibian organs during metamorphosis. Apoptosis 15:350–364
Kamimura M, Shimura S, Kiuchi M (2003) Simple manipulation of silkworm molting by an artificial diet containing plant-derived 20-hydroxyecdysone. J Insect Biotech Seri 72:197–201
Kodama R, Yoshida A, Mitsui T (1995) Programmed cell death at the periphery of the pupal wing of the butterfly, Pieris rapae. Roux’s Archiv Dev Biol 204:418–426
Kourtis N, Tavernarakis N (2009) Autophagy and cell death in model organisms. Cell Death Differ 16:21–30
Lobbia S, Niitsu S, Fujiwara H (2003) Female-specific wing degeneration caused by ecdysteroid in the Tussock Moth, Orgyia recens: Hormonal and developmental regulation of sexual dimorphism. J Insect Sci 3:1–7
Lockshin RA, Zakeri Z (2001) Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol 2:545–550
Macdonald WP, Martin A, Reed RD (2010) Butterfly wings shaped by a molecular cookie cutter: evolutionary radiation of lepidopteran wing shapes associated with a derived Cut/wingless wing margin boundary system. Evol Dev 12:296–304
Malagoli D, Boraldi F, Annovi G, Quaglino D, Ottaviani E (2009) New insights into autophagic cell death in the gypsy moth Lymantria dispar: a proteomic approach. Cell Tissue Res 336:107–118
Matsushima D, Kasahara R, Matsuno K, Aoki F, Suzuki MG (2019) Involvement of ecdysone signaling in the expression of the doublesex gene during embryonic development in the silkworm, Bombyx mori. Sexual Dev 13:151–163. https://doi.org/10.1159/000502361
Meier P, Finch A, Evans G (2000) Apoptosis in development. Nature 407–801
Mizushima N, Levine B (2010) Autophagy in mammalian development and differentiation. Nat Cell Biol 9:823–830
Montero JA, Hurlé JM (2010) Sculpturing digit shape by cell death. Apoptosis 15:365–375
Murillo-Ramos L, Chazot N, Sihvonen P, Õunap E, Jiang N, Han H, Clarke JT, Davis RB, Tammaru T, Wahlberg N (2021) Molecular phylogeny, classification, biogeography and diversification patterns of a diverse group of moths (Geometridae: Boarmiini). Mol Phylogenet Evol 162:107198. https://doi.org/10.1016/j.ympev.2021.107198
Nakajima K, Yaoita Y (2003) Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn 227:246–255
Nardi JB, Godfrey GL, Bergstrom RA (1991) Programmed cell death in the wings of Orgyia leucostigma. J Morphol 209:121–131
Niitsu S (2001) Wing degeneration due to apoptosis in the female of the winter moth, Nyssiodes lefuarius. Entomol Sci 4:1–7
Niitsu S, Lobbia S, Izumi S, Fujiwara H (2008) Female-specific wing degeneration is triggered by ecdysteroid in cultures of wing discs from the bagworm moth, Eumeta variegata (Insecta: Lepidoptera, Psychidae). Cell Tissue Res 333:169–173
Niitsu S, Toga K, Tomizuka S, Maekawa K, Machida R, Kamito T (2014) Ecdysteroid-induced programmed cell death is essential for sex-specific wing degeneration of the wingless-female winter moth. PLoS One 9 Article ID e89435. https://doi.org/10.1371/journal.pone.0089435
Niitsu S, Kamito T (2021) Morphological and histological examination of short-wing formation in the winter moth Protalcis concinnata (Insecta: Lepidoptera, Geometridae). J Morphol 282:160–168
Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF (2010) Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neuro 13:458–466
Rodriguez E Jr, Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, Baskin L (2012) Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation 84:269–279
Schweichel JU, Merker HJ (1973) The morphology of various types of cell death in prenatal tissues. Teratology 7:253–266
Suzanne M, Steller H (2013) Shaping organisms with apoptosis. Cell Death Differ 20:669–675
Xu T, Jiang X, Denton D, Kumar S (2020) Ecdysone controlled cell and tissue deletion. Cell Death Differ 27:1–14
Wahlberg N, Snäll N, Viidalepp J, Ruohomaki K, Tammaru T (2010) The evolution of female flightlessness among Ennominae of the Holarctic forest zone (Lepidoptera, Geometridae). Mol Phyl Evol 55:929–938
Williams CM (1968) Ecdysone and ecdysone analogues: their assay and action on diapausing pupae of the Cynthia silkworm. Biol Bull 134:344–355
Yamamoto S, Sota T (2007) Phylogeny of the Geometridae and evolution of winter moths inferred from a simultaneous analysis of mitochondrial and nuclear genes. Mol Phyl Evol 44:711–723
Zuzarte-Luis V, Hurlé JM (2002) Programmed cell death in the developing limb. Int J Dev Biol 46:871–876
Acknowledgements
We are grateful to Dr. M. Kamimura for gifting us the 20-hydroxyecdysone solution. We also thank Dr. T. Sugimoto for his useful comments in preparing the manuscript. We also thank Dr. M. Kurokawa for kindly supporting our experimental procedures.
Funding
This work was supported by a Grant-in-Aid for Scientific Research C (Grant No. JP19K06791 to S.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article did not use any animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niitsu, S., Kamito, T. Early cellular development induced by ecdysteroid in sex-specific wing degeneration of the wingless female winter moth. Cell Tissue Res 387, 29–38 (2022). https://doi.org/10.1007/s00441-021-03540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03540-6