Abstract
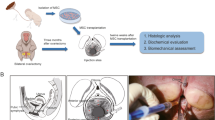
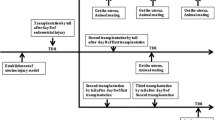
Vaginal structural defects are involved in pelvic organ prolapse (POP). We tested whether mesenchymal stem cell (MSC) therapy can repair the weakened vaginal wall of POP patients as a novel POP treatment. Ninety-six ovariectomized rats were divided into 4 groups (n = 24/group): saline (sal), collagen (col), sal + MSC, and col + MSC groups. Two weeks after ovariectomy, rats received subepithelial injection of 0.3 ml saline, 0.3 ml collagen I gel, and 0.3 ml saline: 3 × 106 human umbilical cord mesenchymal stem cells (HUMSCs), or 0.3 ml collagen I gel: 3 × 106 HUMSCs into the anterior vaginal wall. Eight additional rats underwent in vivo bioluminescence imaging (BLI) to evaluate in vivo cell viability. The BLI signal disappeared within 1 week after MSC injection, and no in vivo MSC differentiation was found. Collagen I content was significantly lower at 4 and 12 weeks in the two MSC groups than in the sal and col groups, while collagen III was significantly higher (P < 0.001). The fraction of smooth muscle in the nonvascular muscularis increased significantly in the two MSC groups at 12 weeks (P < 0.001). ACTA2 mRNA in the col + MSC group was significantly higher than that in the sal group at 2 and 4 weeks (P = 0.042 and P = 0.040). mRNA levels of angiogenic factors (bFGF or VEGF) in the two MSC groups were significantly higher than those in the sal and col groups at different time points. HUMSCs normalized the fibromuscular structures of the vaginal wall of ovariectomized rats potentially through a paracrine effect.





Similar content being viewed by others
References
Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG (2011) Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J 22:789–798
Amin K, Lee U (2019) Surgery for anterior compartment vaginal prolapse: suture-based repair. Urol Clin North Am 46:61–70
Atala A (2012) Tissue engineering of reproductive tissues and organs. Fertil Steril 98:21–29
Barber MD, Maher C (2013) Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J 24:1783–1790
Boennelycke M, Gras S, Lose G (2013) Tissue engineering as a potential alternative or adjunct to surgical reconstruction in treating pelvic organ prolapse. Int Urogynecol J 24:741–747
Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA (2002a) Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol 187:56–63
Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA (2002b) Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol 187:1501–1508
Chen B, Dave B (2014) Challenges and future prospects for tissue engineering in female pelvic medicine and reconstructive surgery. Curr Urol Rep 15:425
Chen G, Wu L, Deng CQ (2011) The effects of BuYang HuanWu decoction and its effective components on proliferation-related factors and ERK1/2 signal transduction pathway in cultured vascular smooth muscle cells. J Ethnopharmacol 135:7–14
Darzi S, Urbankova I, Su K, White J, Lo C, Alexander D, Werkmeister JA, Gargett CE, Deprest J (2016) Tissue response to collagen containing polypropylene meshes in an ovine vaginal repair model. Acta Biomater 39:114–123
DeLancey JO (1993) Pelvic organ prolapse: clinical management and scientific foundations. Clin Obstet Gynecol 36:895–896
Emmerson S, Young N, Rosamilia A, Parkinson L, Edwards SL, Vashi AV, Davies-Tuck M, White J, Elgass K, Lo C, Arkwright J, Werkmeister JA, Gargett CE (2017) Ovine multiparity is associated with diminished vaginal muscularis, increased elastic fibres and vaginal wall weakness: implication for pelvic organ prolapse. Sci Rep 7:45709
Epstein LB, Graham CA, Heit MH (2007) Systemic and vaginal biomechanical properties of women with normal vaginal support and pelvic organ prolapse. Am J Obstet Gynecol 197:165.e1-e6
Fialkow MF, Newton KM, Lentz GM, Weiss NS (2008) Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19:437–440
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN (2010) International Urogyneco-logical Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 29:4–20
Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ (2012) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21:2189–2203
Imoukhuede PI, Popel AS (2012) Expression of VEGF receptors on endothelial cells in mouse skeletal muscle. PLoS One 7:e44791
Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ (2007) Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun 354:700–706
Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369:1027–1038
Jia X, Glazener C, Mowatt G, MacLennan G, Bain C, Fraser C, Burr J (2008) Efficacy and safety of using mesh or grafts in surgery for anterior and/or posterior vaginal wall prolapse: systematic review and meta-analysis. BJOG 115:1350–1361
Kang SK, Kang KS, Jee MK, Kim BS (2010) Vascular endothelial growth factor/kinase insult domain receptor (KDR)/fetal liver kinase 1 (FLK1)-mediated skin- epithelial progenitor cells reprogramming. Tissue Eng Part A 16:2687–2697
Kerkhof MH, Hendriks L, Brolmann HA (2009) Changes in connective tissue in patients with pelvic organ prolapse—a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct 20:461–474
Knuuti E, Kauppila S, Kotila V, Risteli J, Nissi R (2011) Genitourinary prolapse and joint hypermobility are associated with altered type I and III collagen metabolism. Arch Gynecol Obstet 283:1081–1085
Kuismanen K, Sartoneva R, Haimi S, Mannerström B, Tomás E, Miettinen S, Nieminen K (2014) Autologous adipose stem cells in treatment of female stress urinary incontinence: results of a pilot study. Stem Cells Transl Med 3:936–941
Kurpinski K, Chu J, Wang D, Li S (2009) Proteomic profiling of mesenchymal stem cell responses to mechanical strain and TGF-beta1. Cell Mol Bioeng 2:606–614
Li ST (1995) Biologic biomaterials: tissue derived biomaterials (collagen). In: Bronzino JD (ed) The biomedical engineering handbook. CRS Press, Boca Raton, FL, pp 627–647
Mao M, Li Y, Zhang Y, Kang J, Zhu L (2019) Tissue composition and biomechanical property changes in the vaginal wall of ovariectomized young rats. Biomed Res Int 2019:8921284
Moalli PA, Howden NS, Lowder JL, Navarro J, Debes KM, Abramowitch SD, Woo SL (2005) A rat model to study the structural properties of the vagina and its supportive tissues. Am J Obstet Gynecol 192:80–88
Nguyen JN (2008) The use of grafts for anterior vaginal prolapse repair: pros and cons. Curr Opin Obstet Gynecol 20:501–505
Pathi SD, Acevedo JF, Keller PW, Kishore AH, Miller RT, Wai CY, Word RA (2012) Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol 119:134–144
Pokrywczynska M, Jundzill A, Warda K, Buchholz L, Rasmus M, Adamowicz J, Bodnar M, Marszalek A, Helmin-Basa A, Michalkiewicz J, Gagat M, Grzanka A, Frontczak-Baniewicz M, Gastecka AM, Kloskowski T, Nowacki M, Ricordi C, Drewa T (2017) Does the mesenchymal stem cell source influence smooth muscle regeneration in tissue-engineered urinary bladders? Cell Transplant 26:1780–1791
Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA (2008) Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol 198:590.e1–e6
Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258
Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A 103:12167–12172
Sadeghi Z, Isariyawongse J, Kavran M, Izgi K, Marini G, Molter J, Daneshgari F, Flask CA, Caplan A, Hijaz A (2016) Mesenchymal stem cell therapy in a rat model of birth-trauma injury: functional improvements and biodistribution. Int Urogynecol J 27:291–300
Smith FJ, Holman CAJ, Moorin RE, Tsokos N (2010) Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol 116:1096–1100
Takahashi S, Chen Q, Ogushi T, Fujimura T, Kumagai J, Matsumoto S, Hijikata S, Tabata Y, Kitamura T (2006) Periurethral injection of sustained release basic fibroblast growth factor improves sphincteric contractility of the rat urethra denervated by botulinum-a toxin. J Urol 176:819–823
Ulrich D, Edwards SL, Su K, Tan KS, White JF, Ramshaw JAM, Lo C, Rosamilia A, Werkmeister JA, Gargett CE (2014) Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A 20:785–798
Yoshikawa S, Sumino Y, Kwon J, Suzuki T, Kitta T, Miyazato M, Yoshimura N (2017) Effects of multiple simulated birth traumas on urethral continence function in rats. Am J Physiol Renal Physiol 313:F1089–F1096
Zhou L, Lee JH, Wen Y, Constantinou C, Yoshinobu M, Omata S, Chen B (2012) Biomechanical properties and associated collagen composition in vaginal tissue of women with pelvic organ prolapse. J Urol 188:875–880
Acknowledgements
We thank the animal care staff at PUMCH for their assistance in managing the husbandry of the rats used in this study.
Funding
This study was funded by the National Natural Science Foundation of China (81801427, 81771561, 81830043) and the Beijing Municipal Natural Science Foundation (7192154).
Author information
Authors and Affiliations
Contributions
Meng Mao: Protocol development, data collection, data analysis, and manuscript writing. Yaqian Li: animal experiment and data analysis. Ye Zhang: animal experiment. Jia Kang: Data collection. Lan Zhu: Protocol development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, M., Li, Y., Zhang, Y. et al. Human umbilical cord mesenchymal stem cells reconstruct the vaginal wall of ovariectomized Sprague–Dawley rats: implications for pelvic floor reconstruction. Cell Tissue Res 386, 571–583 (2021). https://doi.org/10.1007/s00441-021-03478-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03478-9