Abstract
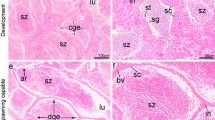
In vertebrates, the primordial germ cells (PGCs) differentiate from extragonadal regions, migrating to gonadal ridge during the embryonic development. However, recent studies in mammals indicate that the PGCs originate from the epiblast and subsequently migrate into the yolk sac. Cell and molecular bases involved in routes during the migration of these cells are still not well understood. Thus, in an attempt to evaluate the participation of matrix metalloproteinases (MMPs) during the gonadal primordium formation in Danio rerio (zebrafish), the route of migration of PGCs was analyzed. In zebrafish, during the migration of the PGCs to the forming gonad, they bind by cytoplasmic processes to the extracellular matrix and migrate through amoeboid movements until they reach the gonadal ridge. During the epiboly, MMPs were not detected. However, after organogenesis, three MMP types were expressed in the somatic cells that were located ahead of the PGCs in the migration route. This expression was maintained throughout the mesentery and was not detected in the PGCs. Upon reaching the gonadal ridge, the PGCs and somatic cells express MMPs and epithelium begins to be formed. After the formation of the basement membrane, the germinal epithelium is delineated by the somatic cells, which remodeling the extracellular matrix. So, a PGC organization occurs through the tissue, forming the gonadal primordium. Concomitantly, granulocytes expressing different MMPs are present. This data in exposing the role of MMPs during the PGC migration to the forming gonad, may point a new way in understanding the reproductive biology of the vertebrates in general.










Similar content being viewed by others
References
Bendel-Stenzel MR, Gomperts M, Anderson R, Heasman J, Wylie C (2000) The role of cadherins during primordial germ cell migration and early gonad formation in the mouse. Mech Dev 91:143–152
Blaser H, Reicman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Kreze L, Heisenberg CP, Raz E (2006) Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell 11:613–627
Braat AK, Speksnijder JE, Zivkovic D (1999a) Germ line development in fishes. Int J Dev Biol 43:45–760
Braat AK, Zandbergen T, Van De Water S, Goos HJ, Zivkovic D (1999b) Characterization of Zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev Dynam 216:153–167
Carvalho L, Heisenberg CP (2010) The yolk syncytial layer in early zebrafish development. Trends Cell Biol 20:586–592
Çek Ş (2006) Early gonadal development and sex differentiation in rosy barb (Puntius conchonius). Anim Biol 56(3):335–350
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Di Carlo A, De Felici M (2000) A role for E-cadherin in mouse primordial germ cell development. Dev Biol 226:209–219
Díez-Torre A, Díaz-Núñez M, Eguizabal C, Silvan U, Arechaga J (2013) Evidence for a role of matrix metalloproteinases and their inhibitors in primordial germ cell migration. Andrology 1(5):779–786
Foyle TP (1993) A histological description of gonadal development and sex differentiation in the salmon (Oncorhynchus kisutch) for both untreated and estradiol immersed fry. J Fish Biol 42:699–712
Fujimoto T, Miayayama T, Fujita M (1977) The origin, migration and fine morphology of human primordial germ cells. Anat Rec 188:315–330
García-Castro M, Anderson R, Heasman J, Wylie C (1997) Interactions between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J Cell Biol 138:471–480
Gevers P, Timmermans LPM (1991) Dye-coupling and the formation and fate of the hypoblast in the teleost fish embryo, Barbus conchonius. Development 112:431–438
Gevers P, Dulos J, Schipper H, Timmermans LPM (1992) Origin of primordial germ cells, as characterized by the presence of nuage in embryos of the teleost fish Barbus conchonius. Eur J Morphol 30:195–204
Gilbert SF, Barresi MJF (2016) Developmental biology, 11th edn. Sinauer Associates, Sunderland
Gomperts M, Garcia-Castro M, Wylie C, Heasman J (1994) Interactions between primordial germ cells play a role in their migration in mouse embryos. Development 120:135–141
Hamaguchi S (1982) A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res 227:139–151
Heasman J, Wylie CC (1981) Contact relations and guidance of primordial germ cells on their migratory route in embryos of Xenopus laevis. Proc R Soc Lond Series B Biol Sci 213:41–58
Ho RK (1992) Cell movements and cell fate during zebrafish gastrulation. Development 116(Supp):65–73
Howe K, Clark MD, Stemple DL (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503
Hulboy DL, Rudolph LA, Matrisian LM (1997) Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 3:27–45
Jaglarz MK, Howard KR (1994) Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development 120:83–89
Janssens E, Gaublomme D, De Groef L, Darras VM, Arckens L, Delorme N, Claes F, Hove IV, Moons L (2013) Matrix metalloproteinase 14 in the zebrafish: an eye on retinal and retinotectal development. PLoS One 8:e52915
Jia R, Nie L-W, Wang N, Wang J (2009) Molecular cloning and expression patterns of the Vasa gene from Rana nigromaculata (Amphibia: Anura). Zoologia 26:316–322
Johanson Z, Underwood C, Richter M (2019) Evolution and development of fishes. Cambridge University Press, New York, USA
Khokha R, Murthy A, Weiss A (2013) Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 13:649–665
Kobayashi T, Kajiura-Kobayashi H, Nagahama Y (2000) Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis of a teleost fish, tilapia, Oreochromis niloticus. Mech Develop 99:139–142
Kunz YW (2004) Developmental biology of teleost fishes. Springer, Dordrecht
Kurilo LF (1981) Oogenesis in antenatal development in man. Hum Genet 57:86–92
Lawson KA, Dunn NR, Roelen BA, Zeinstra L, Davis AM, Wright CVE, Korving JPWFM, Hogan BLM (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13:424–436
Ledda S, Bogliolo L, Bebbere D, Ariu F, Pirino S (2010) Characterization, isolation and culture of primordial germ cells in domestic animals: recente progress and insights from the ovine species. Theriogenology 74:534–543
Li M, Hong N, Xu H, Yi M, Li C, Gui J, Hong Y (2009) Medaka vasa is required for migration but not survival of primordial germ cells. Mech Dev 126:366–381
Mazzoni TS, Quagio-Grassiotto I (2020) In totum immunostaining: a histological analysis tool for small dimensions biological samples. Int J Biol Med Res 11(1):6938–6943
Mazzoni TS, Grier HJ, Quagio-Grassiotto I (2010) Germline cysts and the formation of the germinal epithelium during the female gonadal morphogenesis in Cyprinus carpio (Teleostei: Ostariophysi). Anat Rec 293:1581–1606
Mazzoni TS, Grier HJ, Quagio-Grassiotto I (2014) Male gonadal differentiation and the paedomorphic evolution of the testis in Teleostei. Anat Rec 297:1137–1162
Mazzoni TS, Lo Nostro FL, Antoneli FN, Quagio-Grassiotto I (2018) Action of the metalloproteinases in gonadal remodeling during sex reversal in the sequential hermaphroditism of the teleostei fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells 7:34–60
Molyneaux K, Wylie C (2004) Primordial germ cell migration. Int J Dev Biol 48:537–544
Nakamura M, Takahashi H, Hiroi O (1974) Sex differentiation in the masu salmon (Oncorhynchus masou). Sci Rep Hokkaido Salmon Hatchery 28:1–8
Nakamura M, Kobayashi T, Chang XT, Nagahama Y (1998) Gonadal sex differentiation in teleost fish. J Exp Zool 281:362–372
Otani S, Kitauchi T, Saito T, Sakao S, Maegawa S, Inoue K, Yamaha E (2005) The formation of primordial germ cells from germline cells in spherical embryos derived from the blastodisc of 2-cell embryos in goldfish, Carassius auratus. Int J Dev Biol 49:843–850
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233
Paluch EK, Raz E (2013) The role and regulation of blebs in cell migration. Curr Opin Cell Biol 25:582–590
Patiño R, Takashima F (1995) Gonads. In: Takashima F, Hibiya T (eds) An atlas of fish histology: normal and pathological features, 2nd edn. Gustav Fisher, Tokyo Kodansha Stuttgart, pp 128–153
Quintero-Hunter I, Grier H, Muscato M (1991) Enhancement of histological detail using metanil yellow as counterstain in periodic acid/Schiff’s hematoxylin staining of glycol methacrylate tissue sections. Biotech Histochem 66:169–172
Reichman-Fried M, Minina S, Raz E (2004) Autonomous modes of behavior in primordial germ cell migration. Dev Cell 6:589–596
Ricci JM, Martinez ER, Butzge AJ, Doretto LB, Oliveira MA, Bombardelli RA, Bogerd J, Nóbrega RH (2018) Characterization of vasa homolog in a neotropical catfish, Jundiá (Rhamdia quelen): molecular cloning and expression analysis during embryonic and larval development. Gene 654:116–126
Richardson E, Lehmann R (2010) Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol 11:37–49
Santana JCO, Quagio-Grassiotto I (2014) Extracellular matrix remodeling of the testes through the male reproductive cycle in Teleostei fish. Fish Physiol Biochem 40:1863–1875
Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH (2014) Larsen’s human embryology, 5th, Elsevier edn, Churchill Livingstone
Solnica-Krezel L (2005) Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol 15:R213–R228
Starz-Gaiano M, Lehmann R (2001) Moving towards the next generation. Mech Dev 105:5–18
Uchida D, Yamashita M, Kitano T, Iguchi T (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718
Warga RM, Kimmel CB (1990) Cell movements during epiboly and gastrulation in zebrafish. Development 108:569–580
Weidinger G, Wolke U, Köprunner M, Thisse C, Thisse B, Raz E (2002) Regulation of zebrafish primordial germ cell migration by attraction towards an intermediate target. Development 129:25–36
Wolpert L, Tickle C, Arias AM (2015) Principles of development. Oxford University Press, USA
Yabuta Y, Kurimoto K, Ohinata Y, Seki Y, Saitou M (2006) Gene expression dynamics during germline specification in mice identified by quantitative single-cell gene expression profiling. Biol Reprod 75:705–716
Yamamoto K (1982) Periblast in the egg of the eel, Anguilla japonica. Japan J Ichthyol 28:423–430
Yoon C, Kawakami K, Hopkins N (1997) Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124:3157–3166
Funding
This study was funded by the Brazilian Agency FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo—process number: 2014/00868-3 and 2015/16358-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Ethics Committee on Animal Experimentation of the Institute of Biosciences of Botucatu—number 580-IBB-UNESP.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazzoni, T.S., Quagio-Grassiotto, I. Presence of the matrix metalloproteinases during the migration of the primordial germ cells in zebrafish gonadal ridge. Cell Tissue Res 383, 707–722 (2021). https://doi.org/10.1007/s00441-020-03288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03288-5