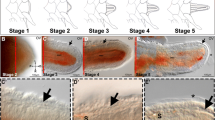
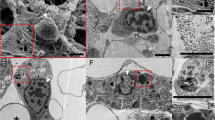
Abstract
Extracellular matrix (ECM) plays a dynamic role during tissue development and re-growth. Body part regeneration efficiency relies also on effective ECM remodelling and deposition. Among invertebrates, echinoderms are well known for their striking regenerative abilities since they can rapidly regenerate functioning complex structures. To gather insights on the involvement of ECM during arm regeneration, the brittle star Amphiura filiformis was chosen as experimental model. Eight ECM genes were identified and cloned, and their spatio-temporal and quantitative expression patterns were analysed by means of whole mount in situ hybridisation and quantitative PCR on early and advanced regenerative stages. Our results show that almost none of the selected ECM genes are expressed at early stages of regeneration, suggesting a delay in their activation that may be responsible for the high regeneration efficiency of these animals, as described for other echinoderms and in contrast to most vertebrates. Moreover, at advanced stages, these genes are spatially and temporally differentially expressed, suggesting that the molecular regulation of ECM deposition/remodelling varies throughout the regenerative process. Phylogenetic analyses of the identified collagen-like genes reveal complex evolutionary dynamics with many rounds of duplications and losses and pinpointed their homologues in selected vertebrates. The study of other ECM genes will allow a better understanding of ECM contribution to brittle star arm regeneration.








Similar content being viewed by others
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, Ath edn. Garland Science, New York, pp 1065–1125
Altenhoff AM, Glover NM, Train C-M, Kaleb K, Vesztrocy AW, Dylus D, de Farias TM, Zile K, Stevenson C, Long J, Redestig H, Gonnet GH, Dessimoz C (2018) The OMA orthology database in 2018: retrieving evolutionary relationships among all domains of life through richer web and programmatic interfaces. Nucleic Acids Res 46:D477–D485
Arpino V, Brock M, Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44-46:247–254
Barros CS, Franco SJ, Müller U (2011) Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol 3:a005108
Bely AE, Nyberg KG (2009) Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol 25:161–170
Ben Amar M, Bianca C (2016) Towards a unified approach in the modeling of fibrosis: a review with research perspectives. Phys Life Rev 17:61–85
Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M (2015a) Wound repair during arm regeneration in the red starfish Echinaster sepositus. Wound Repair Regen 23:611–622
Ben Khadra Y, Ferrario C, Di Benedetto C, Said K, Bonasoro F, Candia Carnevali MD, Sugni M (2015b) Re-growth, morphogenesis, and differentiation during starfish arm regeneration. Wound Repair Regen 23:623–634
Ben Khadra Y, Sugni M, Ferrario C, Bonasoro F, Oliveri P, Martinez P, Candia Carnevali MD (2018) Regeneration in stellate echinoderms: Crinoidea, Asteroidea and Ophiuroidea. In: Kloc M, Kubiak JZ (eds) Marine organisms as model systems in biology and medicine ©Springer International Publishing AG, part of Springer Nature 2018, vol Chapter 14. https://doi.org/10.1007/978-3-319-92486-1_14
Biressi ACM, Zou T, Dupont S, Di Benedetto C, Bonasoro F, Thorndyke MC, Candia Carnevali MD (2010) Wound healing and arm regeneration in Ophioderma longicaudum and Amphiura filiformis (Ophiuroidea, Echinodermata): comparative morphogenesis and histogenesis. Zoomorphology 129:1–19
Blankenship J, Benson S (1984) Collagen metabolism and spicule formation in sea urchin micromere. Exp Cell Res 152:98–104
Bock O, Mrowietz U (2002) Keloids. A fibroproliferative disorder of unknown etiology. Hautarzt 53:515
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801
Bosch TCG (2007) Why polyps regenerate and we don't: towards a cellular and molecular framework for Hydra regeneration. Dev Biol 303:421–433
Brockes JP, Kumar A (2002) Plasticity and reprogramming of differentiated cells in amphibian regeneration. Mol Cell Biol 3:566–574
Brockes JP, Kumar A (2008) Comparative aspects of animal regeneration. Ann Rev Cell Develop Biol 24:525–549
Burns G, Ortega-Martinez O, Thorndyke MC, Peck LS, Dupont S, Clark MS (2011) Dynamic gene expression profiles during arm regeneration in the brittle star Amphiura filiformis. J Exp Mar Biol Ecol 407:315–322
Byrne M (1994) Ophiuroidea. In: Microscopic anatomy of invertebrates, vol 14. Echinodermata, pp 247–343
Cabrera-Serrano A, García-Arrarás JE (2004) RGD-containing peptides inhibit intestinal regeneration in the sea cucumber Holothuria glaberrima. Dev Dyn 231:171–178
Cameron RA, Samanta M, Yuan A, He D, Davidson E (2009) SpBase: the sea urchin database and web site. Nucleic Acids Res D:750–754
Candia Carnevali MD (2006) Regeneration in echinoderms: repair, regrowth and cloning. Invert Surv J:364–376
Chia F-S, Xing J (1996) Echinoderm coelomocytes. Zool Stud 35:231–254
Clouse RM, Linchangco GV Jr, Kerr AM, Reid RW, Janies DA (2015) Phylotranscriptomic analysis uncovers a wealth of tissue inhibitor of metalloproteinases variants in echinoderms. R Soc Open Sci 2:150377
Czarkwiani A, Dylus DV, Oliveri P (2013) Expression of skeletogenic genes during arm regeneration in the brittle star Amphiura filiformis. Gene Expr Patterns 13:464–472
Czarkwiani A, Ferrario C, Dylus DV, Sugni M, Oliveri P (2016) Skeletal regeneration in the brittle star Amphiura filiformis. Front Zool 13:18
Delroisse J, Ullrich-Lüter E, Ortega-Martinez O, Dupont S, Arnone MI, Mallefet J, Flammang P (2014) High opsin diversity in a non-visual infaunal brittle star. BMC Genomics 15:1035
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
Dupont S, Thorndyke MC (2006) Growth or differentiation? Adaptive regeneration in the brittle star Amphiura filiformis. J Exp Biol 209:3873–3881
Dylus DV, Blowes LM, Czarkwiani A, Elphick MR, Oliveri P (2018) Developmental transcriptome of the brittle star Amphiura filiformis reveals gene regulatory network rewiring in echinoderm larval skeleton evolution. Genome Biology 19:26
Erickson JR, Echeverri K (2018) Learning from regeneration research organisms: the circuitous road to scar free wound healing. Dev Biol 433(2):144–154
Exposito J, Valcourt U, Cluzel C, Lethias C (2010) The fibrillar collagen family. IJMS 11(2):407–426
Ferrario C, Ben Khadra Y, Czarkwiani A, Zakrzewski A, Martinez P, Colombo G, Bonasoro F, Candia Carnevali MD, Oliveri P, Sugni M (2018) Fundamental aspects of arm repair phase in two echinoderm models. Dev Biol Spec Issue Regen 433(2):297–309
Geer LY, Domrachev M, Lipman DJ, Bryant SH (2002) CDART: protein homology by domain architecture. Genome Res 12(10):1619–1623
Gelse K, Pöschl E, Aigner T (2003) Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev 55:1531–1546
Gemberling M, Bailey TJ, Hyde DR, Poss KD (2013) The zebrafish as a model for complex tissue regeneration. Trends Genet 29:611-620
Godwin J, Kuraitis D, Rosenthal N (2014) Extracellular matrix considerations for scar-free repair and regeneration: insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol 56:47–55
González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N (2011) Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138:1663–1674
Govindan J, Iovine MK (2015) Dynamic remodeling of the extra cellular matrix during zebrafish fin regeneration. Gene Expr Patterns 19(1–2):21–29
Hernroth B, Farahani F, Brunborg G, Dupont S, Gejmek A, Sköld HN (2010) Possibility of mixed progenitor in sea star arm regeneration. J Exp Zool B Mol Dev Evol 314B:1–12
Hyman LH (1955) The invertebrates. Vol. 4. Echinodermata. Mc Graw Hill Book Company Inc. New York, Toronto, London 34-131, 245–412
Hynes RO (2009) The extracellular matrix: not just pretty fibrils. Science 326:1216–1219
Iorio V, Troughton LD, Hamill KJ (2015) Laminins: roles and utility in wound repair. Adv Wound Care (New Rochelle) 4(4):250–263
Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong S-Y, Lopez R, Hunter S (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics. DOI:https://doi.org/10.1093/bioinformatics/btu031
Karp RD, Coffaro KA (1982) Cellular defense systems of the Echinodermata. Phylogeny and Ontogeny 257–282
Keane TJ, Horejs C-M, Stevens MM (2018) Scarring vs. functional healing: matrix-based strategies to regulate tissue repair. Adv Drug Deliv Rev 129:407–419
King RS, Newmark PA (2012) The cell biology of regeneration JCB: review. J Cell Biol 196:553–562
Kishore U, Reid KB (2000) C1q: structure, function, and receptors. Immunopharmacology 49(1–2):159–170
Kudtarkar P, Cameron RA (2017) Echinobase: an expanding resource for echinoderm genomic information. Database 2017 DOI: https://doi.org/10.1093/database/bax074
Liesi P, Kaakkola S, Dahl D, Vaheri A (1984) Laminin is induced in astrocytes of adult brain by injury. EMBO J 3:683–686
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3(12):a005058
Mashanov VS, Zueva OR, García-Arrarás JE (2014) Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC Genomics 15:357
Mescher AL, Neff AW (2005) Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol 93:39–66
Miao T, Zixuan W, Sun L, Li X, Xing L, Bai Y, Wang F, Yang H (2017) Extracellular matrix remodeling and matrix metalloproteinases (ajMMP-2 like and ajMMP-16 like) characterization during intestine regeneration of sea cucumber Apostichopus japonicus. Comp Biochem Physiol B: Biochem Mol Biol 212:12–23
Milligan M (1946) Trichrome stain for formalin-fixed tissue. Am J Clin Pathol 10:184
Nye HLD, Cameron JA, Chernoff EAG, Stocum DL (2003) Regeneration of the Urodele limb: a review. Dev Dyn 226:280–294
Okazaki K, Inoué S (1976) Crystal property of the larval sea urchin spicule. Develop Growth Differ 18:413–434
Ortiz-Pineda PA, Ramírez-Gómez F, Pérez-Ortiz J, González-Díaz S, Santiago-De Jesús F, Hernández-Pasos J, Del Valle-Avila C, Rojas-Cartagena C, Suárez-Castillo EC, Tossas K, Méndez-Merced AT, Roig-López JL, Ortiz-Zuazaga H, García-Arrarás JE (2009) Gene expression profiling of intestinal regeneration in the sea cucumber. BMC Genomics 10:262. DOI:https://doi.org/10.1186/1471-2164-10-262
Purushothaman S, Saxena S, Meghah V, Brahmendra Swamy CV, Ortega-Martinez O, Dupont S, Idris M (2015) Transcriptomic and proteomic analyses of Amphiura filiformis arm tissue-undergoing regeneration. J Proteome 112:113–124
Quiñones JL, Rosa R, Ruiz DL, García-Arrarás JE (2002) Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol 250:181–197
Rahban SR, Garner WL (2003) Fibroproliferative scars. Clin Plast Surg 30:77
Rousselle P, Montmasson M, Garnier C (2018) Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol 75-76:12-26
Saló E, Abril JF, Adell T, Cebrià F, Eckelt K, Fernàndez-Taboada E, Handberg-Thorager M, Iglesias M, Molina MD, Rodrìguea-Esteban G (2009) Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol 53:1317–1327. https://doi.org/10.1387/ijdb.072414es
Satoh A, Hirata A, Makanae A (2012) Collagen reconstitution is inversely correlated with induction of limb regeneration in Ambystoma mexicanum. Zool Sci 29:191–197
Sheehy EJ, Cunniffe GM, O’Brien FJ (2018) Collagen-based biomaterials for tissue regeneration and repair. Peptides and proteins as biomaterials for tissue regeneration and repair 127-150 DOI:https://doi.org/10.1016/B978-0-08-100803-4.00005-X
Smith VJ (1981) The echinoderms. In: Ratcliffe NA, Rowley AF (eds) Invertebrate blood cells. Academic Press, London, pp 513–562
Stevenson TJ, Vinarsky V, Atkinson DL, Keating MT, Odelberg SJ (2006) Tissue inhibitor of metalloproteinase 1 regulates matrix metalloproteinase activity during newt limb regeneration. Dev Dyn 235(3):606–616
Sun L, Chen M, Yang H, Wang T, Liu B, Shu C, Gardiner DM (2011) Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp Biochem Physiol D. DOI:https://doi.org/10.1016/j.cbd.2011.03.002
Swinehart IT, Badylak SF (2016) Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev Dyn 245:351–360
Tassava RA, Nace JD, Wei Y (1996) Extracellular matrix protein turnover during salamander limb regeneration. Wound Repair Regen 4(1):75–81
Tsonis PA (2000) Regeneration in vertebrates. Dev Biol 221:273–284
Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO (2006) The echinoderm adhesome. Dev Biol 300:252–266
Yi S, Ding F, Gong L, Gu X (2017) Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr Stem Cell Res Ther 12(3):233–246
Yun S (2014) The role of extracellular matrix in planarian regeneration. PhD thesis (University of Hong Kong)
Zhao A, Qin H, Fu X (2016) What determines the regenerative capacity in animals? BioScience 66(9):735–746
Acknowledgements
The authors are grateful to the Sven Lovén Centre for Marine Sciences in Kristineberg (Sweden) for help during the collection of experimental animals. We thank Wendy Hart (University College London) and Fraser Simpson (University College London) for help with gene cloning. We are grateful to Iain C. Wilkie for his suggestions for improving the manuscript. This work was partly funded by the KVA fund SL2015-0048 from the Royal Swedish Academy of Science. CF and LP were funded by an Erasmus Placement fellowship. AC was funded by a Wellcome Trust PhD studentship (099745/Z/12/Z). MS was funded by a Young Researcher Grant of the University of Milan.
Funding
This work was partly funded by the KVA fund SL2015-0048 from the Royal Swedish Academy of Science. CF and LP were funded by an Erasmus Placement fellowship. AC was funded by a Wellcome Trust PhD studentship (099745/Z/12/Z). MS was funded by a Young Researcher Grant of the University of Milan.
Author information
Authors and Affiliations
Contributions
CF, MS and PO conceived the study and wrote the manuscript. CF and AC carried out the molecular experiments and the histological sections. AC provided the experimental animal images. LP helped with in situ hybridisation experiments and performed Q-PCR with PO. DVD performed phylogenetic analysis. CF, AC, DVD, MS, PO and MDCC analysed the data. All authors contributed to and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14.9 mb)
Rights and permissions
About this article
Cite this article
Ferrario, C., Czarkwiani, A., Dylus, D.V. et al. Extracellular matrix gene expression during arm regeneration in Amphiura filiformis. Cell Tissue Res 381, 411–426 (2020). https://doi.org/10.1007/s00441-020-03201-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03201-0