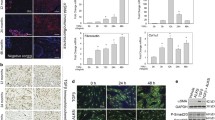
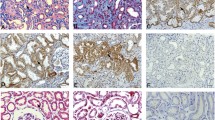
Abstract
Abnormal activation of Wnt signaling has been demonstrated in the wound healing process and the pathogenesis of fibrotic disorders, with Wnt4 specifically identified as having a key role in the pathogenesis of renal, pulmonary and liver fibrosis. Wnt4 also was found to be upregulated by transforming growth factor-β1 (TGF-β1) in fetal and postnatal murine fibroblasts and bone marrow mesenchymal cells, suggesting an underlying cooperation between Wnt4 and TGF-β1 in fibrosis. However, the specific roles of Wnt4 in TGF-β1-induced skin myofibroblast transition and hypertrophic scar formation remain unclear. In the present study, we first observed reduced Wnt4 expression in hypertrophic scar tissue compared with that in normal skin tissue. Following upregulation by TGF-β1, Wnt4 inhibited the TGF-β1-induced transdifferentiation of fibroblasts into myofibroblasts. Using fibroblast-populated collagen lattice contraction assays, we showed that the increased contractility induced by TGF-β1 was significantly blocked by exogenous Wnt4 and the α-smooth muscle actin (α-SMA) expression was decreased in fibroblasts in the collagen lattices. In addition, knockdown of Wnt4 resulted in further increases in α-SMA and collagen I expressions. Further investigation showed that Wnt4 could inhibit the autocrine effect of TGF-β1 as well as block the phosphorylation of Smad3 and ERK but not of AKT or JNK. Lastly, using hypertrophic scar–derived fibroblasts, we showed that the elevated α-SMA and collagen I levels were markedly reduced after treatment with Wnt4. Taken together, our results suggest that Wnt4 negatively regulates TGF-β1-induced fibroblast activation, which may represent a novel therapeutic strategy for the treatment and prevention of hypertrophic scars.





Similar content being viewed by others
References
Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK (2011) Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 4:469–483
Bastakoty D, Young PP (2016) Wnt/beta-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J 30:3271–3284
Bell E, Ivarsson B, Merrill C (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A 76:1274–1278
Castellone MD, Laukkanen MO (2017) TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front Biosci (Schol Ed) 9:31–45
Chen X, Shi C, Meng X, Zhang K, Li X, Wang C, Xiang Z, Hu K, Han X (2016) Inhibition of Wnt/beta-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-beta1 and FGF-2. Exp Mol Pathol 101:22–30
Colwell AS, Krummel TM, Longaker MT, Lorenz HP (2006) Wnt-4 expression is increased in fibroblasts after TGF-beta1 stimulation and during fetal and postnatal wound repair. Plast Reconstr Surg 117:2297–2301
Cutroneo KR (2007) TGF-beta-induced fibrosis and SMAD signaling: oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen 15(Suppl 1):S54–S60
Dabiri G, Tumbarello DA, Turner CE, Van de Water L (2008) Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol 128:2518–2525
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289
DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD (2013) Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24:1399–1412
Fan C, Dong Y, Xie Y, Su Y, Zhang X, Leavesley D, Upton Z (2015) Shikonin reduces TGF-beta1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med 36:985–991
Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN (2016) Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 388:1427–1436
Friedstat JS, Hultman CS (2014) Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 72:S198–S201
George SJ (2009) Regulation of myofibroblast differentiation by convergence of the Wnt and TGF-beta1/Smad signaling pathways. J Mol Cell Cardiol 46:610–611
Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, Murcia C, International Advisory Panel on Scar M (2014) Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg 40:825–831
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61:337–346
He T, Bai X, Yang L, Fan L, Li Y, Su L, Gao J, Han S, Hu D (2015) Loureirin B inhibits hypertrophic scar formation via inhibition of the TGF-beta1-ERK/JNK pathway. Cell Physiol Biochem 37:666–676
Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL (2012) Sustained Wnt/beta-catenin signaling rescues high glucose induction of transforming growth factor-beta1-mediated renal fibrosis. Am J Med Sci 344:374–382
Hsu YC, Chen MJ, Yu YM, Ko SY, Chang CC (2010) Suppression of TGF-beta1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302:717–724
Hutchenreuther J, Leask A (2016) A tale of two orgins: do myofibroblasts originate from different sources in wound healing and fibrosis? Cell Tissue Res 365:507–509
Jiang X, Huang B, Yang H, Li G, Zhang C, Yang G, Lin F, Lin G (2017) TGF-beta1 is involved in vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway. Cell Physiol Biochem 42:2230–2241
Labus MB, Stirk CM, Thompson WD, Melvin WT (1998) Expression of Wnt genes in early wound healing. Wound Repair Regen 6:58–64
Lemoinne S, Thabut D, Housset C (2016) Portal myofibroblasts connect angiogenesis and fibrosis in liver. Cell Tissue Res 365:583–589
Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y (2017) (Pro)renin receptor is an amplifier of Wnt/beta-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol 28:2393–2408
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D (2012) Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci 65:38–49
Lyu J, Joo CK (2006) Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea 25:S24–S28
Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD (2016) Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27:781–790
Mia MM, Bank RA (2016) The pro-fibrotic properties of transforming growth factor on human fibroblasts are counteracted by caffeic acid by inhibiting myofibroblast formation and collagen synthesis. Cell Tissue Res 363:775–789
Popova AP, Bozyk PD, Goldsmith AM, Linn MJ, Lei J, Bentley JK, Hershenson MB (2010) Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am J Phys Lung Cell Mol Phys 298:L735–L743
Rajasekaran MR, Kanoo S, Fu J, Bhargava V, Mittal RK (2017) Wnt-beta catenin signaling pathway: a major player in the injury induced fibrosis and dysfunction of the external anal sphincter. Sci Rep 7:963
Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE (2001) Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 107:69–82
Reddy AT, Lakshmi SP, Zhang Y, Reddy RC (2014) Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J 28:5299–5310
Rezvani M, Espanol-Suner R, Malato Y, Dumont L, Grimm AA, Kienle E, Bindman JG, Wiedtke E, Hsu BY, Naqvi SJ, Schwabe RF, Corvera CU, Grimm D, Willenbring H (2016) In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell 18:809–816
Saito S, Zhuang Y, Shan B, Danchuk S, Luo F, Korfei M, Guenther A, Lasky JA (2017) Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta-PI3K-Akt pathway. PLoS One 12:e0186615
Saitoh A, Hansen LA, Vogel JC, Udey MC (1998) Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp Cell Res 243:150–160
Scharenberg MA, Pippenger BE, Sack R, Zingg D, Ferralli J, Schenk S, Martin I, Chiquet-Ehrismann R (2014) TGF-beta-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J Cell Sci 127:1079–1091
Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, Fang XB, Liu JQ, Tao K, Zhu XX, Tang CW, Hu DH (2013) Protection against TGF-beta1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res 305:341–352
Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Putzer BM, Arauzo-Bravo MJ, Steinemann D, Luedde T, Schwabe RF, Manns MP, Scholer HR, Schambach A, Cantz T, Ott M, Sharma AD (2016) Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell 18:797–808
Sun YB, Qu X, Caruana G, Li J (2016) The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 92:102–107
Surendran K, McCaul SP, Simon TC (2002) A role for Wnt-4 in renal fibrosis. Am J Physiol Ren Physiol 282:F431–F441
Thannickal VJ (2012) Mechanisms of pulmonary fibrosis: role of activated myofibroblasts and NADPH oxidase. Fibrogenesis Tissue Repair 5:S23
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, Ka SM, Tang YJ, Chung AC, To KF, Nikolic-Paterson DJ, Lan HY (2016a) TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 7:8809–8822
Wang Z, Han Z, Tao J, Wang J, Liu X, Zhou W, Xu Z, Zhao C, Ju X, Wang Z, Tan R, Gu M (2016b) Transforming growth factor-beta1 induces endothelial-to-mesenchymal transition via Akt signaling pathway in renal transplant recipients with chronic allograft dysfunction. Ann Transplant 21:775–783
Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, Gottardi CJ, MacDougald OA, Varga J (2011) Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum 63:1707–1717
Wu CS, Wu PH, Fang AH, Lan CC (2012) FK506 inhibits the enhancing effects of transforming growth factor (TGF)-beta1 on collagen expression and TGF-beta/Smad signalling in keloid fibroblasts: implication for new therapeutic approach. Br J Dermatol 167:532–541
Yazdani S, Bansal R, Prakash J (2017) Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev
Zhang J, Li Y, Bai X, Li Y, Shi J, Hu D (2017) Recent advances in hypertrophic scar. Histol Histopathol 33:27–39
Zhong X, Tu YJ, Li Y, Zhang P, Wang W, Chen SS, Li L, Chung AC, Lan HY, Chen HY, Li GS, Wang L (2017) Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): a noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am J Transl Res 9:2920–2932
Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123:1096–1108
Funding
This work was supported by the National Natural Science Foundation of China (No. 81201470, No. 81772071, No. 81501684), the Natural Science Foundation of Shaanxi Province (No. 2017JQ8031) and the China Postdoctoral Science Foundation (No. 2014M562600).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Written informed consent was obtained from each patient.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the experiments, including the collection of skin and scar tissue samples from patients, were approved by the ethics committee of Xijing Hospital, The Fourth Military Medical University (Approval No. KY20183245).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
(PNG 186 kb)
Supplementary Fig. 2
(PNG 94 kb)
Supplementary Fig. 3
(PNG 296 kb)
Supplementary Fig. 4
(PNG 178 kb)
Supplementary Fig. 5
(PNG 152 kb)
Supplementary Fig. 6
(PNG 186 kb)
Supplementary Table 1
(DOC 703 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Zhao, B., Zhu, H. et al. Wnt4 negatively regulates the TGF-β1-induced human dermal fibroblast-to-myofibroblast transition via targeting Smad3 and ERK. Cell Tissue Res 379, 537–548 (2020). https://doi.org/10.1007/s00441-019-03110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03110-x