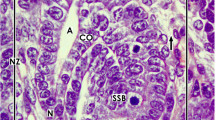
Abstract
In healthy newborn babies, nephrogenesis proceeds unnoticed until birth. With start of the perinatal period, morphogenetic activity in the renal outer cortex consisting of an inner maturation zone and an outer nephrogenic zone is downregulated by unknown signals. One of the results is that the entire nephrogenic zone as well as the contained progenitor cells and niches disintegrate. In contrast, a too early inactivation of the nephrogenic zone takes place in the kidneys of preterm and low birth weight babies. Although they are born in a period of active nephrogenesis, pathological findings show that they evolve to a high incidence oligonephropathy. However, very few data exist about cell biological changes that are evoked by harming, further most of causing molecules, exact cell targets, and related molecular pathways are not identified. Although impairment of nephrogenesis was the subject of research in animal species, there is only limited information available pertaining to the pathological traces in the nephrogenic zone of the human fetal kidney. In this situation, the lack of basic morphological data is particularly aggravating. Surprisingly, there are not even ultrastructural investigations available. Since concrete information is lacking also in relevant textbooks, the current contribution likes to present key features of the nephrogenic zone in the fetal human kidney. Simultaneously, it is a call to explore systematically a hardly known area.







Similar content being viewed by others
References
Abdel-Hakeem AK, Henry TQ, Magee TR, Desal M, Ross MG, Mansano RZ, Torday JS, Nast CC (2008) Mechanisms of impaired nephrogenesis with fetal growth restriction: altered renal transcription and growth factor expression. Am J Obstet Gynecol 199(3):252.e1–252.e7
Abitbol CL, DeFreitas MJ, Strauss J (2016) Assessment of kidney function in preterm infants: lifelong implications. Pediatr Nephrol 31(12):2213–2222
Al-Awqati O, Goldberg MR (1998) Architectural patterens in branching morphogenesis in the kidney. Kidney Int 54(6):1832–1842
Awazu M, Hida M (2015) Maternal nutrition restriction inhibits ureteric bud branching but does not affect the duration of nephrogenesis in rats. Pediatr Res 77(5):633–639
Barnett C, Nnoli O, Abdulmahdi W, Nesi L, Shen M, Zullo JA, Payne DL, Azar T, Dwivedi P, Syed K, Gromis J, Lipphardt M, Jules E, Maranda EL, Patel A, Rabadi MM, Ratcliff BB (2017) Low birth weight is associated with impaired murine kidney development and function. Pediatr Res 82(2):340–348
Benedetto A, Accetta G, Fujita Y, Charras G (2014) Spatiotemporal control of gene expression using microfluids. Lab Chip 14(7):1336–1347
Boivin FJ, Sarin S, Lim J Javidan A, Svajger B, Khalili H, Bridgewater D (2015) Stromally expressed β-catenin modulates Wnt9b signaling in the ureteric epithelium. PLoS One 10(3):e0120347
Brennan S, Kandasamy Y (2017) Ultrasound imaging of the renal parenchyma of premature neonates for the assessment of renal growth and glomerulomegaly. Ultrasound Med Biol 43(11):2546–2549
Bruno S, Chiabotto G, Camussi G (2014) Consise review: different mesenchymal stromal/stem cell populations reside in the adult kidney. Stem Cells Transl Med 3(12):1451–1455
Buchholz B, Schley G, Eckhardt KU (2016) The impact of hypoxia on nephrogenesis. Curr Opin Nephrol Hypertens 25(3):180–186
Callaway DA, McGill-Vargas LL, Quinn A, Jordan JL, Winter LA, Anzueto D, Dick EJ, Blanco CL (2018) Prematurity disrupts glomeruli development while prematurity and hyperglycemia lead to altered nephron maturation and increased oxidative stress in newborn baboos. Pediatr Res 83(3):702–711
Cebrian C, Asai N, D’gati V, Costantini F (2014) The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment. Cell Rep 7(1):127–137
Chi L, Saarela U, Railo A, Prunskaite-Hyyryläinen R, Skovorodkin I et al (2011) A sectreted BMP antagonist, CER1, fine tunes the spatial organization of the ureteric bud tree during mouse kidney development. PLoS One 6(11):e27676
Chung E, Deacon P, Park JS (2017) Notch is required for the formation of all nephron segments and primes nephron progenitors for differentiation. Development 144(24):4530–4539
Combes AN, Davies JA, Little MH (2015) Cell-cell interactions driving kidney morphogenesis. Curr Top Dev Biol 112:467–508
Combes AN, Lefevre JG, Wilson S, Hamilton NA, Little MH (2016) Cap mesenchyme cell swarming during kidney development is influenced by attraction, repulsion, and adhesion to the ureteric tip. Dev Biol 418(2):297–306
Da Sacco S, Thornton ME, Petrosyan A, Lavarreda-Pearce M, Sedrakyan S et al (2017) Direct isolation and characterization of human nephron progenitors. Stem Cells Transl Med 6(2):419–433
Evans RG, Goddard D, Eppel GA, O’Connor PM (2011) Factors that render the kisney susceptible to tissue hypoxia in hypoxemia. Am J Phys Regul Integr Comp Phys 300(4):R931–R940
Fanni D, Sanna A, Gerosa C, Puddu M, Faa G, Fanos V (2015) Each niche has an actor: multiple stem cell niches in the preterm kidney. Ital J Pediatr 41:78
Fanos V, Castagnola M, Faa G (2015) Prolonging nephrogenesis in preterm infants: a new approach for prevention of kidney disease in adulthood? Iran J Kidney Dis 9(3):180–185
Gerl K, Steppan D, Fuchs M, Wagner C, William C, Kurtz A, Kurt B (2017) Activation of hypoxia signaling in stromal progenitors impairs kidney development. Am J Pathol 187(7):1496–1511
Gerosa C, Fanni D, Faa A, Van Eyken P, Ravarino A, Fanos V, Faa G (2017) Low vascularization of the nephrogenic zone of the fetal kidney suggests a major role for hypoxia in human nephrogenesis. Int Urol Nephrol 49(9):1621–1616
Girardi A, Raschi E, Galletti S, Poluzzi E, Faldella G, Allegaert K, De Ponti F (2015) Drug-induced renal damage in preterm neonates: state of the art and methods for early detection. Drug Saf 38(6):535–551
Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ (2009) Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Ren Physiol 297(6):F1668–F1677
Han KH, Lim JM, Kim MY, Kim H, Madsen KM et al (2005) Expression of endothelial nitric oxide synthase in developing rat kidney. Am J Physiol Ren Physiol 288(4):F694–F702
Harvey SJ (2012) Models for studies of proteoglycans in kidney pathophysiology. Methods Mol Biol 836:259–284
Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E (1996) Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged Helix transcription factor BF-2. Genes Dev 10(12):1467–1478
Hemker SL, Sims-Lucas S, Ho J (2016) Role of hypoxia during nephrogenesis. Pediatr Nephrol 31(10):1571–1577
Hendry C, Rumballe B, Moritz K, Little MH (2011) Defining and redefining the nephron progenitor population. Pediatr Nephrol 26(9):1395–1406
Hirakawa Y, Mizukami K, Yoshihara T, Takahashi I, Khulan P, Honda T, Mimura I, Tanaka T, Tobita S, Nangaku M (2018) Intravital phophrence lifetime imaging of the renal cortex accurately measures renal hypoxia. Kidney Int 93(6):1483–1489
Holthöfer H (1987) Vascularization of the embryonic kidney. Detection of endothelial cells with Ulex europaeus I lectin. Cell Differ 20(1):27–31
Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S (2014) Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9(2):e88400
Isaacson D, Shen J, McCreedy D, Calvert M, McDevitt T, Cunha G, Baskin L (2018) Lightsheet fluorescence microscopy of branching human kidney. Kidney Int 93(2):525
Jastrow H (2018) Anatomisches Institut, Johannes Gutenberg Universität, Mainz: Gefässe der Niere: http://www.drjastrow.de/WAI/EM/EMNiere.html
Kandasamy Y, Smith R, Wright IM (2012) Oligonephropathy of prematurity. Am J Perinatol 29(2):115–120
Kandasamy Y, Smith R, Wright IM, Lumbers ER (2013) Extra-uterine renal growth in preterm infants: oligonephropathy and prematurity. Pediatr Nephrol 28(9):1791–1796
Kloth S, Aigner J, Brandt E, Moll R, Minuth WW (1993) Histochemical markers reveal an unexpected heterogeneous composition of the renal embryonic collecting duct epithelium. Kidney Int 44(3):527–536
Kloth S, Ebenbeck C, Monzer J, de Vries U, Minuth WW (1997) Three-dimensional organization of the developing vasculature of the kidney. Cell Tissue Res 287(1):193–201
Kobayashi K (1978) Fine structure of the mammalian renal capsule: the atypical smooth muscle cell and its functional meaning. Cell Tissue Res 195(3):381–394
Kobayashi H, Liu J, Urrutia AA, Burmakin M, Ishii K, Rajan M, Davidoff O, Saifudeen Z, Haase VH (2017) Hypoxia-inducible factor prolyl-4-hydroxylation in FOXD1 lineage cells is essential for normal kidney development. Kidney Int 92(6):1370–1383
Lefevre J, Short KM, Lamberton TO, Michos O, Graf D, Smyth IM, Hamilton NA (2017) Branching morphogenesis in the developing kidney is governed by rules that pattern the ureteric tree. Development 144(23):4377–4385
Leuning DG, Engelse MA, Lievers E, Bijkerk R, Reinders MEJ, de Boer HC, van Kooten C, Rabelink TJ (2017) The human kidney capsule contains a functionally distinct mesenchymal stromal cell population. PLoS One 12(12):e0187118
Levinson RS, Batourina E, Choi C, Vorontchiikhina M, Kitajewski J, Mendelsohn CL (2005) Fox1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132(3):529–539
Li W, Hartwig S, Rosenblum ND (2014) Developmental origins and functions of stromal cells in the normal and diseased mammalian kidney. Dev Dyn 243:853–863
Lindström NO, Guo J, Kim AD, Tran T, Guo Q, De Sena Brandine G, Ransick A, Parvez RK, Thornton ME, Baskin L, Grubbs B, McMahon JA, Smith AD, McMahon AP (2018a) Conserved and different features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J Am Soc Nephrol 29(3):806–824
Lindström NO, Tran T, Guo J, Rutledge E, Parvez RK, Thornton ME, Grubbs B, McMahon JA, McMahon AP (2018b) Conserved and different molecular and anatomic features of human and mouse nephron patterning. J Am Soc Nephrol 29(3):825–840
Lindström NO, McMahon JA, Guo J, Tran T, Rutledge E, Parvez RK, Saribekyan G, Schuler RE, Liao C, Kim AD, Abdelhalim A, Ruffins SW, Thornton ME, Baskin L, Kesselmann C, McMahon AP (2018c) Conserved and different features of human and mouse kidney organogenesis. J Am Soc Nephrol 29(3):785–805
Luyckx VA (2017) Preterm birth and its impact on renal health. Semin Nephrol 37(4):311–319
Magella B, Adam M, Potter AS, Venkatasubramanian M, Chetal K, Hay SB, Salomonis N, Potter SS (2018) Cross-platform single cell analysis of kidney development shows stromal cells express GDNF. Dev Biol 434(1):36–47
Mao Y, Francis-West P, Irvine KD (2015) Fat4/Dchs1 signaling between stromal and cap mesenchyme cells influences nephrogenesis and ureteric bud branching. Development 142(15):2574–2585
Mari C, Winyard P (2015) Concise review: understanding the renal progenitor cell niche in vivo to recapitulate nephrogenesis in vitro. Stem Cells Transl Med 4:1463–1471
Menendez-Castro C, Nitz D, Cordasic N, Jordan J, Bäuerle T, Fahlbusch FB, Rascher W, Hilgers KF, Hartner A (2018) Neonatal nephron loss during active nephrogenesis – detrimental impact with long-term renal consequences. Sci Rep 8(1):4542
Minuth WW (2017) Concepts for a therapeutic prolongation of nephrogenesis in preterm and low birth weight babies must correspond to structural-functional properties in the nephrogenic zone. Mol Cell Pediatr 4(1):12
Minuth WW (2018a) Structural and functional links between capsule and nephrogenic zone in fetal human kidney. J Pediatric Neonatal Individualized Medicine (JPNIM) (in press)
Minuth WW (2018b) Reading first coordinates from the nephrogenic zone in human fetal kidney. Nephron 138(2):137–146
Minuth WW (2018c) Action plan for prolongation of nephrogenesis in preterm and growth restricted babies: explore ultrastructure of the nephrogenic zone, identify a molecular target, select a viable drug and find a path for administration. Drug Res 68(1):5–16
Minuth WW, Denk L (2014) Structural links between the renal stem/progenitor cell niche and the organ capsule. Histochem Cell Biol 141(5):458–471
Minuth WW, Denk L (2015) Advanced fixation for transmission electron microscopy unveils special extracellular matrix within the renal stem/progenitor cell niche. Methods Mol Biol 12:21–37
Minuth WW, Denk L (2016) What is the functional background of filigree extracellular matrix and cell-cell connections at the interface of the renal stem/progenitor cell niche? J Pediatric and Neonatal Individualized Medicine (JPNIM) 5(1):e50115
Molema G, Aird WC (2012) Vascular heterogeneity in the kidney. Semin Nephrol 32(2):145–155
Morizane R, Bonventre JY (2017) Kidney organoids: a translational journey. Trends Mol Med 23(3):246–263
Munro DAD, Hohenstein P, Davies JA (2017a) Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci Rep 7(1):3273
Munro DAD, Hohenstein P, Coate TM, Davies JA (2017b) Refuting the hypothesis that semaphorin-3f/neuropilin-2 exclude blood vessels from the cap mesenchyme in the developing kidney. Dev Dyn 246(12):1047–1056
Muthukrishnan SD, Ryzhov S, Karolak M, Oxburgh L (2018) Nephron progenitor cell death elicits a limited compensatory response associated with interstitial expansion in neonatal kidney. Dis Model Mech 11(1). https://doi.org/10.1242/dmm.030544
Nagalakshimi VK, Yu J (2015) The ureteric bud epithelium: morphogenesis and roles in metanephric kidney patterning. Mol Reprod Dev 82:151–166
Nemolato S, Cabras T, Messana I, Gerosa C, Faa G, Castanola M (2014) Do betathymosins play a role in human nephrogenesis? In: Faa G, Fanos V (eds) Kidney development in renal pathology. Current Clinical Pathology, Humana Press - Springer, New York, pp 81–93
Nguyen MU, Wallace MJ, Pepe S, Menheniott TR, Moss TJ, Burgner D (2015) Perinatal inflammation: a common factor in the early origins of cardiovascular disease? Clin Sci (Lond) 129(8):769–784
Nishita M, Qiao S, Miyamoto M, Okinaka Y, Yamada M, Hashimoto R, Iijama K, Otani H, Hartmann C, Nishinakamura R, Minami Y (2014) Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding. Mol Cell Biol 34(16):3096–3105
O’Brien LL, Guo Q, Lee Y, Tran T, Benazet JD, Whitney PH, Valouev A, McMahon AP (2016) Differential regulation of mouse and human nephron progenitors by the six family of transcriptional regulators. Development 143(4):595–608
O’Connor PM (2006) Renal oxygen delivery: mating delivery to meatbolic demand. Clin Exp Pharmacol Physiol 33(10):961–967
Oxburgh L, Muthukrishnan SD, Brown A (2017) Growth factor regulation in the nephrogenic zone of the developing kidney. Results Probl Cell Differ 60:137–164
Park HC, Yasuda K, Kuo MC, Ni J, Ratliff B, Chander P, Goligorsky MS (2010) Renal capsule as stem cell niche. Am J Physiol Renal Physiol 298(5):F1254–F1262
Phua YL, Chu JY, Marrone AK, Bodnar AJ, Sims-Lucas S, Ho J (2015) Renal stromal miRNAs are required for normal nephrogenesis and glomerular mesangial survival. Physiol Rep 3(10). https://doi.org/10.14814/phy2.e12537
Qiu L, Hyink DP, Gans WH, Amsler K, Wilson PD, Burrow CR (2004) Midkine promotes selective expansion of the nephrogenic mesenchyme during kidney organogenesis. Organogenesis 1(1):14–21
Riccio P, Cebrian C, Zong H, Hippenmeyer S, Constantini F (2016) Ret and Etv promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol 14(6):e1002382
Roker LA, Nemri K, Yu J (2017) Wnt7b signaling from the ureteric bud epithelium regulates medullary capillary development. J Am Soc Nephrol 28(1):250–259
Rosenblum ND (2008) Developmental biology of the human kidney. Semin Fetal Neonatal Med 13:125–132
Rowan CJ, Sheybani-Deloui S, Rosenblum ND (2017) Origin and function of the renal stroma in health and disease. Results Probl Cell Differ 60:2015–2229
Rowan CJ, Li W, Martiirosyan H, erwood S, Hu D, Kim YK, sheybani-Deloui S, Mulder J, Blake J, chen L, Rosenblum ND (2018) Hedgehog-GLI signaling in Foxd1-positive stromal cells promotes murine nephrogenesis via TGFβ signaling. Development 145(13). https://doi.org/10.1242/dev.159947
Rumballe BA, Georgas KM, Combes AN, Ju AL, Gilbert T, Little MH (2011) Nephron formation adopts a novel spatial topology at cessation of neohrogenesis. Dev Biol 360(1):110–122
Rusu MC, Mogoanta L, Pop F, Dobra MA (2018) Molecular phenotypes in the human kidney: myoid stromal cells/telocytes and myoepithelial cells. Ann Anat 218:95–104
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ (2017) Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27:275–283
Rymer C, Paredes J, Halt K, Schaefer C, Wiersch J et al (2014) Renal blood flow and oxygenation drive nephron progenitor differentiation. Am J Physiol Ren Physiol 307(3):F337–F345
Saboktakin MR, Tabatabaei RM (2015) Supramolecular hydrogels as drug delivery systems. Int J Biol Macromol 75:426–436
Saifudeen Z, Dipp S, Stefkova J, Yao X, Lookabaugh S, El-Dahr SS (2009) P53 regulates metanephroc development. J Am Soc Nephrol 20(11):2328–2337
Schreuder MF, Bueters RR, Allegaert K (2014) The interplay between drugs and the kidney in premature neonates. Pediatr Nephrol 29(11):2083–2091
Song R, Janssen A, Li Y, El-Dahr S, Yosypiv IV (2017a) Prorenin receptor controls renal branching morphogenesis via Wnt/β-catenin signaling. Am J Physiol Ren Physiol 312(3):F407–F417
Song R, Lopez MLSS, Yosypiv IV (2017b) Foxd1 is an upstream regulator of the renin-angiotensin system during metanephric kidney development. Pediatr Res 82(5):855–862
Song R, Kidd L, Janssen A, Yosypiv IV (2018) Conditional ablation of the prorenin receptor in nephron progenitor cells results in developmental programming of hypertension. Physiolol Rep 6(7):e13644
Stritzke A, Thomas S, Amin H, Fusch C, Lodha A (2017) Renal consequences of preterm birth. Mol Cell Pediatr 4(1):2
Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RSC, Hoy WE, Bertram JF, Black MJ (2011) Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22:1365–1374
Sutherland MR, Bertagnolli M, Lucaszewski MA, Huyard F, Yzydorczyk C, Luu TM, Nuyt AM (2014) Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension 63(1):12–18
Tran C, Damaser MS (2015) Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev 82-83:1–11
Vignais ML, Caicedo A, Brondello JM, Jorgensen C (2017) Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target metabolism, homeostasis, and response to therapy. Stem Cells Int. https://doi.org/10.1155/2017/6917941
Wang J, Wang F, Wang Z, Li S, Chen L, Liu C, Sund D (2018) Protective effect of GDNF-engineered amniotic fluid-derived stem cells on the renal ischaemia reperfusion injury in vitro. Cell Prolif 51(2):e12400
Whitehouse T, Stotz M, Taylor V, Stidwill R, Singer M (2006) Tissue oxygen and hemodynamics in renal medulla, cortex, and corticomedullary junction during hemorrhage-reperfusion. Am J Phys 291(3):F647–F653
Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R (2001) Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49(4):460–467
Funding
The project was supported by the Emeriti Research Fund, University of Regensburg, D-93053 Regensburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minuth, W.W. Key features of the nephrogenic zone in the fetal human kidney—hardly known but relevant for the detection of first traces impairing nephrogenesis. Cell Tissue Res 375, 589–603 (2019). https://doi.org/10.1007/s00441-018-2937-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2937-4