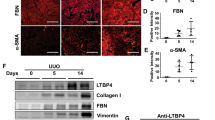
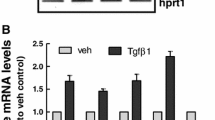
Abstract
Renal tubular epithelial cells actively contribute to the development of renal fibrosis and may be targeted by anti-fibrotic drugs. Relaxin-2 (RLX2) applied as recombinant protein is suggested to be renoprotective. Therefore, we investigated whether human primary tubular epithelial cells (hPTEC) obtained from various donors were target cells for the anti-fibrotic actions of RLX2. Treatment of hPTEC with RLX2 reduced the TGF-β1-induced secretion of the pro-fibrotic factor CTGF (connective tissue growth factor) and inhibited fibronectin synthesis and secretion. Furthermore, metalloproteinase MMP2 secretion was increased, with no effect on MMP9. Considerable differences were observed between hPTEC obtained from different donors. Therefore, expression of the relaxin family peptide receptor RXFP1, the major mediator of renal RLX2 effects, was analyzed. A validated antibody detected a double band of 80–90 kDa in cellular homogenates by Western blotting. Expression of the detected protein was not altered by incubation with TGF-β1 and RLX2-induced modulation of CTGF expression did not correlate with the putative receptor expression. Therefore, relaxin family receptors RXFP1–4 were assessed by RNA-seq analysis. No evidence was found for mRNA expression of any of these receptors in several hPTEC preparations. Lack of RXFP1 mRNA was confirmed by qPCR using mRNA obtained from THP-1 cells as positive control. Our data thus provide evidence for primary renal human tubular epithelial cells as targets for the anti-fibrotic actions of RLX2. However, anti-fibrotic effects were observed at micromolar concentrations of RLX2 and shown to be independent of RXFP1 expression.





Similar content being viewed by others
References
Ball DK, Moussad EE, Rageh MA, Kemper SA, Brigstock DR (2003) Establishment of a recombinant expression system for connective tissue growth factor (CTGF) that models CTGF processing in utero. Reproduction 125:271–284
Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ (2013) Relaxin family peptides and their receptors. Physiol Rev 93:405–480
Bogzil AH, Ashton N (2009) Relaxin-induced changes in renal function and RXFP1 receptor expression in the female rat. Ann N Y Acad Sci 1160:313–316
Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD, Samuel CS (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86:75–85
Chuang PY, Menon MC, He JC (2013) Molecular targets for treatment of kidney fibrosis. J Mol Med (Berl) 91:549–559
Cosen-Binker LI, Binker MG, Cosen R, Negri G, Tiscornia O (2006) Relaxin prevents the development of severe acute pancreatitis. World J Gastroenterol 12:1558–1568
Dschietzig T, Bartsch C, Stangl V, Baumann G, Stangl K (2004) Identification of the pregnancy hormone relaxin as glucocorticoid receptor agonist. FASEB J 18:1536–1538
Dschietzig T, Bartsch C, Baumann G, Stangl K (2009) RXFP1-inactive relaxin activates human glucocorticoid receptor: further investigations into the relaxin-GR pathway. Regul Pept 154:77–84
Dschietzig TB, Krause-Relle K, Hennequin M, von Websky K, Rahnenfuhrer J, Ruppert J, Gron HJ, Armbruster FP, Bathgate RA, Aschenbach JR, Forssmann WG, Hocher B (2015) Relaxin-2 does not ameliorate nephropathy in an experimental model of type-1 diabetes. Kidney Blood Press Res 40:77–88
Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjostedt E, Lundberg E, Szigyarto CA, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson A, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlen M (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13:397–406
Figueiredo KA, Mui AL, Nelson CC, Cox ME (2006) Relaxin stimulates leukocyte adhesion and migration through a relaxin receptor LGR7-dependent mechanism. J Biol Chem 281:3030–3039
Garber SL, Mirochnik Y, Brecklin CS, Unemori EN, Singh AK, Slobodskoy L, Grove BH, Arruda JA, Dunea G (2001) Relaxin decreases renal interstitial fibrosis and slows progression of renal disease. Kidney Int 59:876–882
Ghosh RK, Banerjee K, Tummala R, Ball S, Ravakhah K, Gupta A (2017) Serelaxin in acute heart failure: most recent update on clinical and preclinical evidence. Cardiovasc Ther 35:55–63
Giehl K, Keller C, Muehlich S, Goppelt-Struebe M (2015) Actin-mediated gene expression depends on RhoA and Rac1 signaling in proximal tubular epithelial cells. PLoS One 10:e0121589
Goppelt-Struebe M, Schaefer D, Habenicht AJ (1997) Differential regulation of cyclooxygenasse-2 and 5-lipoxygenase-activating protein (FLAP) expression by glucocorticoids in monocytic cells. BrJPharmacol 122:619–624
Grampp S, Platt JL, Lauer V, Salama R, Kranz F, Neumann VK, Wach S, Stohr C, Hartmann A, Eckardt KU, Ratcliffe PJ, Mole DR, Schodel J (2016) Genetic variation at the 8q24.21 renal cancer susceptibility locus affects HIF binding to a MYC enhancer. Nat Commun 7:13183
Halls ML, Bathgate RA, Sutton SW, Dschietzig TB, Summers RJ (2015) International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacol Rev 67:389–440
Heeg MH, Koziolek MJ, Vasko R, Schaefer L, Sharma K, Muller GA, Strutz F (2005) The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int 68:96–109
Horton JS, Yamamoto SY, Bryant-Greenwood GD (2011) Relaxin modulates proinflammatory cytokine secretion from human decidual macrophages. Biol Reprod 85:788–797
Hossain MA, Rosengren KJ, Haugaard-Jonsson LM, Zhang S, Layfield S, Ferraro T, Daly NL, Tregear GW, Wade JD, Bathgate RA (2008) The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J Biol Chem 283:17287–17297
Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol 14:1257–1271
Keller C, Kroening S, Zuehlke J, Kunath F, Krueger B, Goppelt-Struebe M (2012) Distinct mesenchymal alterations in N-cadherin and e-cadherin positive primary renal epithelial cells. PLoS One 7:e43584
Knier B, Cordasic N, Klanke B, Heusinger-Ribeiro J, Daniel C, Veelken R, Hartner A, Hilgers KF (2011) Effect of the plasminogen-plasmin system on hypertensive renal and cardiac damage. J Hypertens 29:1602–1612
Kroening S, Neubauer E, Wessel J, Wiesener M, Goppelt-Struebe M (2009) Hypoxia interferes with connective tissue growth factor (CTGF) gene expression in human proximal tubular cell lines. Nephrol Dial Transplant 24:3319–3325
Kroening S, Neubauer E, Wullich B, Aten J, Goppelt-Struebe M (2010) Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: modulation by hypoxia. Am J Physiol Renal Physiol 298:F796–F806
Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M, Conrad KP, Parry LJ (2017) Vascular actions of relaxin: nitric oxide and beyond. Br J Pharmacol 174:1002–1014
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, Tregear GW, Samuel CS (2009) Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J 23:1219–1229
Muehlich S, Cicha I, Garlichs CD, Krueger B, Posern G, Goppelt-Struebe M (2007) Actin-dependent regulation of connective tissue growth factor (CTGF). Am J Physiol Cell Physiol 292:1732–1738
Muehlich S, Rehm M, Ebenau A, Goppelt-Struebe M (2017) Synergistic induction of CTGF by cytochalasin D and TGFbeta-1 in primary human renal epithelial cells: role of transcriptional regulators MKL1, YAP/TAZ and Smad2/3. Cell Signal 29:31–40
Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP (2006) Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 20:2352–2362
Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M (2003) Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton. J Biol Chem 278:44305–44311
Preisser F, Giehl K, Rehm M, Goppelt-Struebe M (2016) Inhibitors of oxygen sensing prolyl hydroxylases regulate nuclear localization of the transcription factors Smad2 and YAP/TAZ involved in CTGF synthesis. Biochim Biophys Acta 1863:2027–2036
Ramazani Y, Knops N, Elmonem MA, Nguyen TQ, Arcolino FO, van den Heuvel L, Levtchenko E, Kuypers D, Goldschmeding R (2018) Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol 68–69:44–66
Samarakoon R, Goppelt-Struebe M, Higgins PJ (2010) Linking cell structure to gene regulation: signaling events and expression controls on the model genes PAI-1 and CTGF. Cell Signal 22:1413–1419
Samuel CS, Royce SG, Hewitson TD, Denton KM, Cooney TE, Bennett RG (2017) Anti-fibrotic actions of relaxin. Br J Pharmacol 174:963–976
Samuel CS, Summers RJ, Hewitson TD (2016) Antifibrotic actions of Serelaxin - new roles for an old player. Trends Pharmacol Sci 37:485–497
Shannon S, Vaca C, Jia D, Entersz I, Schaer A, Carcione J, Weaver M, Avidar Y, Pettit R, Nair M, Khan A, Foty RA (2015) Dexamethasone-mediated activation of fibronectin matrix assembly reduces dispersal of primary human glioblastoma cells. PLoS One 10:e0135951
Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF (1992) The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci 33:2242–2250
Sun YB, Qu X, Caruana G, Li J (2016) The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 92:102–107
Vega G, Alarcon S, San Martin R (2016) The cellular and signalling alterations conducted by TGF-beta contributing to renal fibrosis. Cytokine 88:115–125
Viedt C, Burger A, Hansch GM (1995) Fibronectin synthesis in tubular epithelial cells: up-regulation of the EDA splice variant by transforming growth factor beta. Kidney Int 48:1810–1817
Wong SE, Samuel CS, Kelly DJ, Zhang Y, Becker GJ, Hewitson TD (2013) The anti-fibrotic hormone relaxin is not Reno-protective, despite being active, in an experimental model of type 1 diabetes. Protein Pept Lett 20:1029–1038
Xie XC, Cao Y, Yang X, Xu QH, Wei W, Wang M (2017a) Relaxin attenuates contrast-induced human proximal tubular epithelial cell apoptosis by activation of the PI3K/Akt signaling pathway in vitro. Biomed Res Int 2017:2869405
Xie XC, Zhao N, Xu QH, Yang X, Xia WK, Chen Q, Wang M, Fei X (2017b) Relaxin attenuates aristolochic acid induced human tubular epithelial cell apoptosis in vitro by activation of the PI3K/Akt signaling pathway. Apoptosis 22:769–776
Yang DH, Kim HS, Wilson EM, Rosenfeld RG, Oh Y (1998) Identification of glycosylated 38-kDa connective tissue growth factor (IGFBP-related protein 2) and proteolytic fragments in human biological fluids, and up-regulation of IGFBP-rP2 expression by TGF-beta in Hs578T human breast cancer cells. J Clin Endocrinol Metab 83:2593–2596
Zhou D, Liu Y (2016) Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol 12:68–70
Zuehlke J, Ebenau A, Krueger B, Goppelt-Struebe M (2012) Vectorial secretion of CTGF as a cell-type specific response to LPA and TGF-beta in human tubular epithelial cells. Cell Commun Signal 10:25
Acknowledgements
The expert technical assistance of A. Ebenau and M. Rehm is highly appreciated. We are grateful to B. Wullich and his team, Department of Urology, University of Erlangen-Nürnberg, for providing us with kidney tissue.
Funding
This study was supported by a grant from Novartis Pharma GmbH, Nürnberg, Germany. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Grampp, S., Goppelt-Struebe, M. Receptor-independent modulation of TGF-β-induced pro-fibrotic pathways by relaxin-2 in human primary tubular epithelial cells. Cell Tissue Res 374, 619–627 (2018). https://doi.org/10.1007/s00441-018-2904-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2904-0