Abstract
In vitro-generation of β-cells from Wharton’s jelly mesenchymal stem cells (WJ-MSCs) could provide a potential basis for diabetes mellitus cell therapy. However, the generation of functional insulin-producing cells (IPCs) from WJ-MSCs remains a challenge. Recently, obestatin, a gut hormone, was found to promote β-cell generation from pancreatic precursor cells. Accordingly, we hypothesize that obestatin can induce the differentiation of WJ-MSCs into IPCs. Therefore, the purpose of the current study is to examine the ability of obestatin to generate IPCs in comparison to well-known extrinsic factors that are commonly used in IPCs differentiation protocols from MSCs, namely exendin-4 and glucagon-like peptide-1 (GLP-1). To achieve our aims, WJ-MSCs were isolated, cultured and characterized by immunophenotyping and adipocytes differentiation. Afterwards, WJ-MSCs were induced to differentiate into IPCs using two differentiation protocols incorporating either exendin-4, GLP-1 or obestatin. The pancreatic progenitor marker, nestin and β-cell differentiation markers were assessed by qRT-PCR, while the functionality of the generated IPCs was assessed by glucose-stimulated insulin secretion (GSIS). Our results showed that WJ-MSCs exhibit all the characteristics of MSCs. Interestingly, using obestatin in both the short and long differentiation protocols managed to induce the expression of β-cell markers, similar to exendin-4. In GSIS, IPCs generated using either GLP-1 or obestatin showed higher secretion of insulin as compared to those generated using exendin-4 under low-glucose conditions but failed to show a significant response to increased glucose. These results indicate obestatin can be considered as a novel potential factor to consider for generation of IPCs from WJ-MSCs.



Similar content being viewed by others
Abbreviations
- GLP-1:
-
Glucagon like peptide-1
- GLP-1R:
-
Glucagon-like peptide-1 coupled receptors
- GPR-39:
-
Glucagon like peptide receptor-39
- IPCs:
-
Insulin-producing cells
- iPSCs:
-
induced pluripotent stem cells
- MSCs:
-
Mesenchymal stem cells
- T1DM:
-
Type 1 diabetes mellitus
- WJ:
-
Wharton’s jelly
References
Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G (2010) New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev 19:423–438
Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G (2011) Wharton's jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev 7:342–363
Baragli A, Grande C, Gesmundo I, Settanni F, Taliano M, Gallo D, Gargantini E, Ghigo E, Granata R (2013) Obestatin enhances in vitro generation of pancreatic islets through regulation of developmental pathways. PLoS ONE 8:e64374
Bhandari DR, Seo KW, Sun B, Seo MS, Kim HS, Seo YJ, Marcin J, Forraz N, Roy HL, Larry D, Colin M, Kang KS (2011) The simplest method for in vitro beta-cell production from human adult stem cells. Differentiation 82:144–152
Chen L, Jiang X, Yang L (2004) Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 10:3016–3020
Cogger K, Nostro MC (2015) Recent advances in cell replacement therapies for the treatment of type 1 diabetes. Endocrinology 1:8–15
Corrao S, La Rocca G, Lo Iacono M, Corsello T, Farina F, Anzalone R (2013) Umbilical cord revisited: from Wharton's jelly myofibroblasts to mesenchymal stem cells. Histol Histopathol 28:1235–1244
Crevecoeur I, Rondas D, Mathieu C, Overbergh L (2015) The beta-cell in type 1 diabetes: what have we learned from proteomic studies? Proteomics Clin Appl 9:755–766
Depoortere I, Thijs T, Moechars D, De Smet B, Ver Donck L, Peeters TL (2008) Effect of peripheral obestatin on food intake and gastric emptying in ghrelin-knockout mice. Br J Pharmacol 153:1550–1557
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Huang YC, Parolini O, La Rocca G, Deng L (2012) Umbilical cord versus bone marrow-derived mesenchymal stromal cells. Stem Cells Dev 21:2900–2903
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S (2007) Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 25:2837–2844
Kassem DH, Kamal MM, El-Kholy Ael L, El-Mesallamy HO (2016a) Association of expression levels of pluripotency/stem cell markers with the differentiation outcome of Wharton's jelly mesenchymal stem cells into insulin producing cells. Biochimie 127:187–195
Kassem DH, Kamal MM, El-Kholy Ael L, El-Mesallamy HO (2016b) Exendin-4 enhances the differentiation of Wharton's jelly mesenchymal stem cells into insulin-producing cells through activation of various beta-cell markers. Stem Cell Res Ther 7:108
Kim SY, Lee SH, Kim BM, Kim EH, Min BH, Bendayan M, Park IS (2004) Activation of nestin-positive duct stem (NPDS) cells in pancreas upon neogenic motivation and possible cytodifferentiation into insulin-secreting cells from NPDS cells. Dev Dyn 230:1–11
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasa L, Cappello F, Zummo G, Farina F (2009) Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131:267–282
La Rocca G, Lo Iacono M, Corsello T, Corrao S, Farina F, Anzalone R (2013) Human Wharton's jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: new perspectives for cellular therapy. Curr Stem Cell Res Ther 8:100–113
Malgieri A, Kantzari E, Patrizi MP, Gambardella S (2010) Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med 3:248–269
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834
Nekoei SM, Azarpira N, Sadeghi L, Kamalifar S (2015) In vitro differentiation of human umbilical cord Wharton’s jelly mesenchymal stromal cells to insulin producing clusters. World J Clin Cases 3:640–649
Parekh VS, Joglekar MV, Hardikar AA (2009) Differentiation of human umbilical cord blood-derived mononuclear cells to endocrine pancreatic lineage. Differentiation 78:232–240
Park D, Xiang AP, Mao FF, Zhang L, Di CG, Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, Walton N, Lahn BT (2010) Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 28:2162–2171
Precechtelova J, Borsanyiova M, Sarmirova S, Bopegamage S (2014) Type I diabetes mellitus: genetic factors and presumptive Enteroviral etiology or protection. J Pathogens 2014:738512
Qu H, Liu X, Ni Y, Jiang Y, Feng X, Xiao J, Guo Y, Kong D, Li A, Li X, Zhuang X, Wang Z, Wang Y, Chang Y, Chen S, Kong F, Zhang X, Zhao S, Sun Y, Xu D, Wang D, Zheng C (2014) Laminin 411 acts as a potent inducer of umbilical cord mesenchymal stem cell differentiation into insulin-producing cells. J Transl Med 12:135
Rizzo M, Nikolic D, Banach M, Patti AM, Montalto G, Rizvi AA (2014) Incretin-based therapies, glucometabolic health and endovascular inflammation. Curr Pharmaceut Des 20:4953–4960
Santos TM, Percegona LS, Gonzalez P, Calil A, Corradi Perini C, Faucz FR, Camara NO, Aita CA (2010) Expression of pancreatic endocrine markers by mesenchymal stem cells from human umbilical cord vein. Transpl Proc 42:563–565
Seshareddy K, Troyer D, Weiss M (2008) Method to isolate mesenchymal-like cells from Wharton's jelly of umbilical cord. Methods Cell Biol 86:101–119
Shao S, Fang Z, Yu X, Zhang M (2009) Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun 384:401–404
Wang HW, Lin LM, He HY, You F, Li WZ, Huang TH, Ma GX, Ma L (2011) Human umbilical cord mesenchymal stem cells derived from Wharton's jelly differentiate into insulin-producing cells in vitro. Chin Med J 124:1534–1539
Wang H, Jiang Z, Li A, Gao Y (2012) Characterization of insulin-producing cells derived from PDX-1-transfected neural stem cells. Mol Med Rep 6:1428–1432
Watson N, Divers R, Kedar R, Mehindru A, Borlongan MC, Borlongan CV (2014) Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy 17:18–24
Wu X, Liu C, Xu K, Mao X, Zhu J, Jiang J, Cui D, Zhang M, Xu Y, Liu C (2007) Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol 13:3342–3349
Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, Koizumi M, Sone H, Nakata M, Kakei M, Dezaki K (2014) Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Diabetes Obes Metab 16 Suppl 1:111–117
Funding
This research was partially funded by Ain Shams University, Cairo, Egypt as one of the Applied Researches Grants supported by the University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflict of interest.
Electronic supplementary material
Supplemental Figure 1
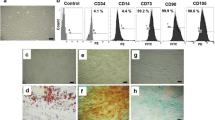
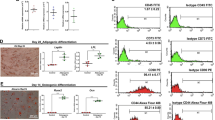
Images of cells generated by either short or long differentiation protocols: Short protocol: (a) control cells, (b) exendin-4, (c) GLP-1 and (d)obestatin. Long protocol: (e) control cells, (f) exendin-4, (g) GLP-1 and (h) obestatin. Scale bar = 50 μm. (GIF 476 kb)
Rights and permissions
About this article
Cite this article
El-Asfar, R.K., Kamal, M.M., Abd EL-Razek, R.S. et al. Obestatin can potentially differentiate Wharton’s jelly mesenchymal stem cells into insulin-producing cells. Cell Tissue Res 372, 91–98 (2018). https://doi.org/10.1007/s00441-017-2725-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2725-6