Abstract
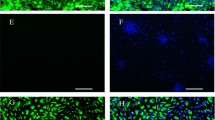
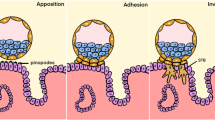
This study was conducted to develop an in vitro model using rat uterine explants to explore complex uterine functions. Rat uterine explants (1–2 mm) were isolated, cultured and further characterized. Steroid hormone treatment of cultured explants showed that both Muc1 and Pr were significantly up-regulated (P < 0.05) by E2. Areg was significantly up-regulated (P < 0.05) by P4 and Igfbp1 was significantly up-regulated (P < 0.05) by the combination of E2 and P4, although, in rat, Igfbp1 is E2-dependent. In vitro decidualization of cultured explants was induced and two potential markers of decidualization, Prl8a2 and Bmp2, were examined. Real-time quantitative PCR data revealed that both Prl8a2 and Bmp2 were significantly up-regulated (P < 0.05) in MPA- and db-cAMP-treated explants compared to the control group of explants. Then, an individual hatched blastocyst and cultured explant was placed in a 96-well (round-bottom U-shaped) plate. Co-culture results showed that stable attachments were observed after 48 h, where embryos were stably attached to the explants and could not be dislodged after mild shaking and/or pipetting. The rates of attachment of embryos to the explants were increased significantly in the P4-treated group (63.6%) compared to the control group (35.5%), after steroid hormone treatment. The rates of attachment were reduced significantly in the E2-treated group (0.0%), where no stable attachments were observed. Despite the necessity of comprehensive investigation, our results suggest that the cultured rat uterine explants can be a useful in vitro model to study uterine functions and early implantation.






Similar content being viewed by others
References
Armant DR, Kaplan HA, Lennarz WJ (1986) Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev Biol 116:519–523
Aronica SM, Katzenellenbogen BS (1991) Progesterone receptor regulation in uterine cells: stimulation by estrogen, cyclic adenosine 3′, 5′-monophosphate, and insulin-like growth factor I and suppression by antiestrogens and protein kinase inhibitors. Endocrinology 128:2045–2052
Bowen JA, Bazer FW, Burghardt RC (1996) Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 55(5):1098–1106
Cao YJ, Zhang CY, Duan EK (1999) The action of fibronectin to development and implantation of mouse blastocysts in vitro. Acta Zool Sin 45:187–193
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K (2000) Embryo implantation. Dev Biol 223:217–237
Cowan S, Calder AA, Kelly RW (2004) Decidualisation of cervical stromal cells. Eur J Obstet Gynecol Reprod Biol 114:189–196
Croze F, Kennedy TG, Schroedter IC, Friesen HG, Murphy LJ (1990) Expression of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in the rat uterus during decidualization. Endocrinology 127(4):1995–2000
Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK (1995) Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9(6):691–705
Davis MW, Vacanti JP (1996) Toward development of an implantable tissue engineered liver. Biomaterials 17:365–372
Ghahary A, Luo J, Murphy LJ (1993) Expression and regulation of insulin-like growth factor binding protein-I in the rat uterus throughout the estrous cycle. Mol Cell Biochem 124(1):43–49
Glenister T (1961) Organ culture as a new method for studying the implantation of mammalian blastocysts. Proc R Soc Lond B 154:428–431
Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE (2001) Developmental biology of uterine glands. Biol Reprod 65(5):1311–1323
Grümmer R, Hohn HP, Mareel MM, Denker HW (1994) Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three- dimensional organ culture system. Placenta 15:411–429
Haimovici F, Anderson DJ (1993) Effects of growth factors and growth factor extracellular matrix interactions on mouse trophoblast outgrowth in vitro. Biol Reprod 49:124–130
Huang JR, Tseng L, Bischof P, Janne OA (1987) Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology 121:2011–2017
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21:2529–2543
Ing NH, Tornesi MB (1997) Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod 56(5):1205–1215
Irwin JC, Kirk D, King RJ (1989) Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril 52:761–768
Islam MR, Yamagami K, Yoshii Y, Yamauchi N (2016) Growth factor induced proliferation, migration, and lumen formation of rat endometrial epithelial cells in vitro. J Reprod Dev 62(3):271–278
Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ (2010) Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 83(3):396–403
Kusama K, Yoshie M, Tamura K, Daikoku T, Takarada T, Tachikawa E (2014) Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 147(6):897–906
L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA (1998) A completely biological tissue-engineered human blood vessel. FASEB J 12:47–56
Landgren BM, Johannisson E, Stavreus-Evers A, Hamberger L, Eriksson H (1996) A new method to study the process of implantation of a human blastocyst in vitro. Fertil Steril 65(5):1067–1070
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Lee DS, Yanagimoto Ueta Y, Suzuki H (2006) Expression of amphiregulin during the pre- and post-implantation period in the mouse reproductive tract. J Reprod Dev 52(6):781–787
Lee KY, DeMayo FJ (2004) Animal models of implantation. Reproduction 128:679–695
Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ (2007) Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27(15):5468–5478
Lindenberg S, Hyttel P, Sjogren A (1989) A comparative study of attachment of human, bovine, and mouse blastocysts to uterine epithelial mono-layer. Hum Reprod 4:446–456
Lindenberg S, Nielsen MH, Lenz S (1985) In vitro studies of human blastocyst implantation. Ann N Y Acad Sci 42:368–374
Lindenberg S, Sundberg K, Kimber SJ (1988) The milk oligosaccharide, lacto-N-fucopentaose I, inhibits attachment of mouse blastocysts on endometrial monolayers. J Reprod Fertil 83:149–165
Ma WG, Song H, Das SK, Paria BC, Dey SK (2003) Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A 100(5):2963–2968
Mak IY, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, White JO (2002) Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 87:2581–2588
Matsui N, Kawano Y, Nakamura S, Miyakawa I (2004) Changes in vascular endothelial growth factor production associated with decidualization by human endometrial stromal cells in vitro. Acta Obstet Gynecol Scand 83:138–143
Matsumoto K, Yamauchi N, Watanabe R, Oozono S, Kubota K, Nishimura K, Wood C, Soh T, Kizaki K, Hattori MA (2009) In vitro decidualization of rat endometrial stromal cells. Cell Tissue Res 335(3):575–583
Parhar RS, Kennedy TG, Lala PK (1988) Suppression of lymphocyte allo-reactivity by early gestational human decidua. Cell Immunol 116:392–410
Paria BC, Reese J, Das SK, Dey SK (2002) Deciphering the cross-talk of implantation: advances and challenges. Science 296:2185–2188
Patrick CW Jr, Mikos AG, McIntire LV (1998) Prospectus of tissue engineering. In: Frontiers in tissue engineering (Eds) Patrick CW Jr, Mikos AG, McIntire LV. Elsevier, New York, pp 3–14
Romagnano L, Bablarz B (1993) Mechanisms of murine trophoblast interaction with laminin. Biol Reprod 49:374–380
Sakkas D, Trounson AO (1990) Coculture of mouse embryos with oviduct and uterine cells prepared from mice at different days of pseudopregnancy. J Reprod Fertil 90:109–118
Salamonsen LA (1999) Role of proteases in implantation. Rev Reprod 4:11–22
Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE (2008) Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod 79(6):1226–1236
Sharkey A (1998) Cytokines and implantation. Rev Reprod 3:52–61
Shiotani M, Noda Y, Mori T (1993) Embryo-dependent induction of uterine receptivity assessed by an in vitro model of implantation in mice. Biol Reprod 49(4):794–801
Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD (1995) Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136(8):3639–3647
Tan Y, Tan D, He M, Gu M, Wang Z, Zeng G, Duan E (2005) A model for implantation: coculture of blastocysts and uterine endometrium in mice. Biol Reprod 72(3):556–561
Teklenburg G, Macklon NS (2009) Review: in vitro models for the study of early human embryo-endometrium interactions. Reprod Sci 16(9):811–818
Yallampalli C, Rajaraman S, Nagamani M (1993) Insulin-like growth factor binding proteins in the rat uterus and their regulation by oestradiol and growth hormone. J Reprod Fertil 97(2):501–505
Yamagami K, Yamauchi N, Kubota K, Nishimura S, Chowdhury VS, Yamanaka K, Takahashi M, Tabata S, Hattori MA (2014) Expression and regulation of Foxa2 in the rat uterus during early pregnancy. J Reprod Dev 60(6):468–475
Yamamoto Y, Kobayashi Y, Okuda K (2014) Purified culture systems for bovine oviductal stromal cells. J Reprod Dev 60:73–77
Yamauchi K, Yamauchi N, Yamagami K, Nakamura N, Yamashita S, Islam MR, Tabata S, Yahiro K, Tamura T, Hashizume K, Hattori MA (2015) Development of an in vitro model for the analysis of bovine endometrium using simple techniques. Anim Sci J 86(5):523–531
Yamauchi N, Takezawa T, Kizaki K, Herath CB, Hashizume K (2003a) Proliferative potential of endometrial stromal cells, and endometrial and placental expression of cyclin in the bovine. J Reprod Dev 49(6):553–560
Yamauchi N, Yamada O, Takahashi T, Imai K, Sato T, Ito A, Hashizume K (2003b) A three-dimensional cell culture model for bovine endometrium: regeneration of a multicellular spheroid using ascorbate. Placenta 24:258–269
Ye TM, Pang RT, Leung CO, Liu W, Yeung WS (2012) Development and characterization of an endometrial tissue culture model for study of early implantation events. Fertil Steril 98(6):1581–1589
Zeng GQ, Cao JY (1996) The study of the coculture of mouse blastocysts on uterine epithelial monolayer. Acta Zool Sin 42:212–217
Zhang CY, Chen YZ, Cao YQ (1996) The coculture of rabbit blastocysts and its endometrial stromal cell. Acta Zool Sin 2:108–109
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Grant support
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (grant no. 26292141).
Additional information
Summary sentence
Rat uterine explants were cultured and characterized. Cultured explants responses to steroid hormones were also investigated. Furthermore, artificial induction of decidualization was studied. Then, the co-culture of hatched blastocyst and cultured explant was initiated and steroid hormones influencing the rates of attachment were investigated.
Rights and permissions
About this article
Cite this article
Islam, M.R., Ikeguchi, Y., Yamagami, K. et al. Development of an in vitro model to study uterine functions and early implantation using rat uterine explants. Cell Tissue Res 370, 501–512 (2017). https://doi.org/10.1007/s00441-017-2679-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2679-8