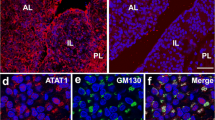
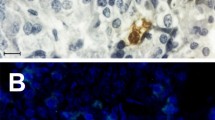
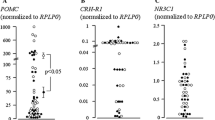
Abstract
The production and secretion of adrenocorticotropin, a proopiomelanocortin (POMC)-derived hormone, by corticotrophs in the anterior pituitary, is regulated by corticotrophin-releasing hormone (CRH) and glucocorticoids. We have previously demonstrated that adrenalectomy induces α-tubulin N-acetyltransferase 1 (ATAT1) expression and α-tubulin acetylation in corticotrophs. However, the regulatory mechanism of ATAT1 expression and the function of acetylated microtubules in corticotrophs are unclear. Here, we analyze the effect of CRH or dexamethasone on Atat1 expression in the mouse corticotroph AtT20 cell line. The expression of Atat1 was increased by CRH and decreased by dexamethasone in AtT20 cells. We examined the effect of Atat1 knockdown on the expression of POMC-associated genes and the dexamethasone-induced nuclear translocation of glucocorticoid receptor (GR) by real-time polymerase chain reaction and Western blot analysis, respectively. Atat1 knockdown resulted in a significant increase in the expression of ACTH-producing genes and decreased the dexamethasone-induced nuclear translocation of GR accompanied with a reduction in α-tubulin acetylation. Atat1 overexpression resulted in a significant increase in α-tubulin acetylation and the dexamethasone-induced nuclear translocation of GR. These results suggest that the acetylated microtubules function as the rail-line for the transportation of GR into the nucleus. We conclude that ATAT1 finely tunes the cellular responses of corticotrophs to hormonal stimulation through an intracellular feedback circuit.






Similar content being viewed by others
References
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J (2010) MEC-17 is an α-tubulin acetyltransferase. Nature 467:218–222
Alper JD, Decker F, Agana B, Howard J (2014) The motility of axonemal dynein is regulated by the tubulin code. Biophys J 107:2872–2880
Birnberg NC, Lissitzky JC, Hinman M, Herbert E (1983) Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci U S A 80:6982–6986
Bloomquist BT, Eipper BA, Mains RE (1991) Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol 5:2014–2024
Cawley NX, Li Z, Loh YP (2016) 60 YEARS OF POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol 56:T77–T97
Coombes C, Yamamoto A, McClellan M, Reid TA, Plooster M, Luxton GWG, Alper J, Howard J, Gardner MK (2016) Mechanism of microtubule lumen entry for the α-tubulin acetyltransferase enzyme αTAT1. Proc Natl Acad Sci U S A 113:E7176–E7184
Czar MJ, Owens-Grillo JK, Yem AW, Leach KL, Deibel JMR, Welsh MJ, Pratt WB (1994) The hsp56 immunophilin component of untransformed steroid receptor complexes is localized both to microtubules in the cytoplasm and to the same nonrandom regions within the nucleus as the steroid receptor. Mol Endocrinol 8:1731–1741
Dompierre JP, Godin JD, Charrin BC, Cordelières FP, King SJ, Humbert S, Saudou F (2007) Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci 27:3571–3583
Drouin J (2016) 60 YEARS OF POMC: transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol 56:T99–T112
Drouin J, Nemer M, Charron J, Gagner JP, Jeannotte L, Sun YL, Therrien M, Tremblay Y (1989) Tissue-specific activity of the pro-opiomelanocortin (POMC) gene and repression by glucocorticoids. Genome 31:510–519
Eberwine JH, Roberts JL (1984) Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem 259:2166–2170
Fink G, Robinson IC, Tannahill LA (1988) Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol (Lond) 401:329–345
Gagner JP, Drouin J (1985) Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol 40:25–32
Grad I, Picard D (2007) The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol 275:2–12
Harrell JM, Murphy PJM, Morishima Y, Chen H, Mansfield JF, Galigniana MD, Pratt WB (2004) Evidence for glucocorticoid receptor transport on microtubules by dynein. J Biol Chem 279:54647–54654
Iredale PA, Duman RS (1997) Glucocorticoid regulation of corticotropin-releasing factor1 receptor expression in pituitary-derived AtT-20 cells. Mol Pharmacol 51:794–799
Jingami H, Matsukura S, Numa S, Imura H (1985) Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/β-lipotropin precursor mRNA in the pituitary in rats. Endocrinology 117:1314–1320
Kovacs JJ, Murphy PJM, Gaillard S, Zhao X, Wu J-T, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao T-P (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607
Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E (2002) Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase a, and MAPK pathways. Mol Endocrinol 16:1638–1651
Krebs A, Goldie KN, Hoenger A (2004) Complex formation with kinesin motor domains affects the structure of microtubules. J Mol Biol 335:139–153
Li L, Yang X-J (2015) Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci 72:4237–4255
Li Y, Shin D, Kwon SH (2013) Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J 280:775–793
Liu JP, Nakakura T, Tomura H, Tobo M, Mogi C, Wang JQ, He XD, Takano M, Damirin A, Komachi M, Sato K, Okajima F (2010) Each one of certain histidine residues in G-protein-coupled receptor GPR4 is critical for extracellular proton-induced stimulation of multiple G-protein-signaling pathways. Pharmacol Res 61:499–505
Maira M, Martens C, Philips A, Drouin J (1999) Heterodimerization between members of the nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol 19:7549–7557
Mano-Otagiri A, Nemoto T, Yamauchi N, Kakinuma Y, Shibasaki T (2016) Distribution of corticotrophin-releasing factor type 1 receptor-like immunoreactivity in the rat pituitary. J Neuroendocrinol. doi:10.1111/jne.12440
Nakakura T, Mogi C, Tobo M, Tomura H, Sato K, Kobayashi M, Ohnishi H, Tanaka S, Wayama M, Sugiyama T, Kitamura T, Harada A, Okajima F (2012) Deficiency of proton-sensing ovarian cancer G protein-coupled receptor 1 attenuates glucose-stimulated insulin secretion. Endocrinology 153:4171–4180
Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H (2015) The elongation of primary cilia via the acetylation of α-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med Mol Morphol 48:44–53
Nakakura T, Nemoto T, Suzuki T, Asano-Hoshino A, Tanaka H, Arisawa K, Nishijima Y, Kiuchi Y, Hagiwara H (2016a) Adrenalectomy facilitates ATAT1 expression and α-tubulin acetylation in ACTH-producing corticotrophs. Cell Tissue Res 366:363–370
Nakakura T, Suzuki T, Nemoto T, Tanaka H, Asano-Hoshino A, Arisawa K, Nishijima Y, Kiuchi Y, Hagiwara H (2016b) Intracellular localization of α-tubulin acetyltransferase ATAT1 in rat ciliated cells. Med Mol Morphol 49:133–143
Park JJ, Loh YP (2008) How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol 22:2583–2595
Perdiz D, Mackeh R, Poüs C, Baillet A (2011) The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal 23:763–771
Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J (1997) Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17:5946–5951
Portran D, Schaedel L, Xu Z, Thery M, Nachury MV (2017) Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol 19:391398
Pratt WB, Galigniana MD, Harrell JM, DeFranco DB (2004) Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal 16:857–872
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16:2166–2172
Satou K, Mochimaru Y, Nakakura T, Kusada T, Negishi J, Musha S, Yoshimura N, Kato Y, Tomura H (2017) Easy detection of hormone secretion from LβT2 cells by using Gaussia luciferase. J Reprod Dev 63:199–204
Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV (2010) The major α-tubulin K40 acetyltransferase αTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A 107:21517–21522
Song Y, Brady ST (2015) Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol 25:125–136
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Szyk A, Deaconescu Alexandra M, Spector J, Goodman B, Valenstein Max L, Ziolkowska Natasza E, Kormendi V, Grigorieff N, Roll-Mecak A (2014) Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157:1405–1415
Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ (1996) Modulation of CRF-R1 mRNA in rat anterior pituitary by dexamethasone: correlation with POMC mRNA. Peptides 17:435–441
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists (B) (16 K18982) to T. N. from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by a grant from the Yuasa Memorial Foundation and by a research grant from Teikyo University School of Medicine. We thank Dr. Masayoshi Iizuka (Teikyo University School of Medicine, Tokyo, Japan) for excellent technical advice and Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure summary
The authors have nothing to disclose.
Electronic supplementary material
Supplemental Fig. 1
Acetylation levels of HSP90 (a) and cytoplasmic dynein (b) in siAtat1- and NT-transfected AtT20 cells. GR has been reported to form a protein complex with HSP90 (a chaperone protein) and cytoplasmic dynein (Grad and Picard 2007). Although HSP90 is deacetylated by HDAC6 (Li et al. 2013), whether ATAT1 is involved in HSP90 acetylation remains unclear. We examined the acetylation level of HSP90 in siAtat1- and NT-transfected AtT20 cells by Western blot analysis. a Acetylated and total HSPs were detected with rabbit anti-acetylated HSP90 (1:2500; 600–401-981, Rockland, Pa., USA) and mouse anti-HSP90 (1:2500; ADI-SPA-830, Enzo life science, N.Y., USA), respectively. Atat1 knockdown showed no effect on both acetylation and expression level of HSP90. b As cytoplasmic dynein functions as a motor protein for the transport of the GC/GR complex from cytoplasm to nucleus along MTs, we compared the cytoplasmic dynein levels in siAtat1- and NT-transfected cells by Western blot analysis with mouse anti-dynein (1:5000; 14–9772-82; eBioscience, Calif., USA). The level of cytoplasmic dynein was the same in both treatment groups. (GIF 37 kb)
Rights and permissions
About this article
Cite this article
Nakakura, T., Suzuki, T., Torii, S. et al. ATAT1 is essential for regulation of homeostasis-retaining cellular responses in corticotrophs along hypothalamic-pituitary-adrenal axis. Cell Tissue Res 370, 169–178 (2017). https://doi.org/10.1007/s00441-017-2654-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2654-4