Abstract
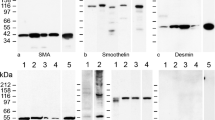
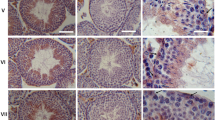
The wall of the seminiferous tubule in rodents consists of an inner layer of myoid cells covered by an outer layer of endothelial cells. Myoid cells are a type of smooth muscle cell containing α-actin filaments arranged in two independent layers that contract when stimulated by endothelin-1. The irregular surface relief of the tubular wall is often considered a hallmark of contraction induced by a variety of stimuli. We examine morphological changes of the rat seminiferous tubule wall during contraction by a combination of light, confocal, transmission and scanning electron microscopy. During ET-1-induced contraction, myoid cells changed from a flat to a conical shape, but their actin filaments remained in independent layers. As a consequence of myoid cell contraction, the basement membrane became wavy, orientation of collagen fibers in the extracellular matrix was altered and the endothelial cell layer became folded. To observe the basement of the myoid cell cone, the endothelial cell monolayer was removed by collagenase digestion prior to SEM study. In contracted tubules, it is possible to distinguish cell relief: myoid cells have large folds on the external surface oriented parallel to the tubular axis, whereas endothelial cells have numerous cytoplasmic projections facing the interstitium. The myoid cell cytoskeleton is unusual in that the actin filaments are arranged in two orthogonal layers, which adopt differing shapes during contraction with myoid cells becoming cone-shaped. This arrangement impacts on other components of the seminiferous tubule wall and affects the propulsion of the tubular contents to the rete testis.










Similar content being viewed by others
References
Clark RV (1976) Three-dimensional organization of testicular interstitial tissue and lymphatic space in the rat. Anat Rec 184:203–25
Dym M, Fawcett DW (1970) The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 3:308–26
Fawcett DW, Heidger PM, Leak LV (1969) Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil 19:109–19
Fernández D, Bertoldi MV, Gómez L et al (2008) Identification and characterization of myosin from rat testicular peritubular myoid cells. Biol Reprod 79:1210–8
Filippini A, Tripiciano A, Palombi F et al (1993) Rat testicular myoid cells respond to endothelin: characterization of binding and signal transduction pathway. Endocrinology 133:1789–96
Kormano M, Hovatta O (1972) Contractility and histochemistry of the myoid cell layer of the rat seminiferous tubules during postnatal development. Z Anat Entwicklungsgesch 137:239–48
Leeson CR, Forman DE (1981) Postnatal development and differentiation of contractile cells within the rabbit testis. J Anat 132:491–511
Leeson CR, Leeson TS (1963) The postnatal development and differentiation of the boundary tissue of the seminiferous tubule of the rat. Anat Rec 147:243–59
Losinno AD, Morales A, Fernández D, Lopez LA (2012) Peritubular myoid cells from rat seminiferous tubules contain actin and myosin filaments distributed in two independent layers. Biol Reprod 86(150):1–8
Maekawa M, Nagano T, Murakami T (1994) Comparison of actin-filament bundles in myoid cells and Sertoli cells of the rat, golden hamster and mouse. Cell Tissue Res 275:395–8
Maekawa M, Kamimura K, Nagano T (1996) Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol 59:1–13
Murakami M, Hamasaki M, Okita S, Abe J (1979) SEM surface morphology of the contractile cells in the rat seminiferous tubules. Experientia 35:1099–101
Nixon GF, Mignery GA, Somlyo AV (1994) Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J Muscle Res Cell Motil 15:682–700
Palombi F, Salanova M, Tarone G et al (1992) Distribution of beta 1 integrin subunit in rat seminiferous epithelium. Biol Reprod 47:1173–82
Rasband W (1997-2006) ImageJ. US National Institut of Health. Bethesda (MD). Avaible from http://rsb.info.nih.gov/ij/
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–12
Romano F, Tripiciano A, Muciaccia B et al (2005) The contractile phenotype of peritubular smooth muscle cells is locally controlled: possible implications in male fertility. Contraception 72:294–7
Ross MH (1967) The fine structure and development of the peritubular contractile cell component in the seminiferous tubules of the mouse. Am J Anat 121:523–57
Smith U, Ryan JW, Michie DD, Smith DS (1971) Endothelial projections as revealed by scanning electron microscopy. Science 173:925–7
Söderström K-O (2009) Scanning Electron microscopy of mechanically isolated seminiferous tubules of the Rat testis. Andrologia 13:155–160
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Tripiciano A, Filippini A, Giustiniani Q, Palombi F (1996) Direct visualization of rat peritubular myoid cell contraction in response to endothelin. Biol Reprod 55:25–31
Tripiciano A, Palombi F, Ziparo E, Filippini A (1997) Dual control of seminiferous tubule contractility mediated by ETA and ETB endothelin receptor subtypes. FASEB J 11:276–86
Tripiciano A, Peluso C, Morena AR et al (1999) Cyclic expression of endothelin-converting enzyme-1 mediates the functional regulation of seminiferous tubule contraction. J Cell Biol 145:1027–38
Tung PS, Fritz IB (1990) Characterization of rat testicular peritubular myoid cells in culture: alpha-smooth muscle isoactin is a specific differentiation marker. Biol Reprod 42:351–65
Virtanen I, Kallajoki M, Närvänen O et al (1986) Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215:10–20
Vogl AW, Soucy LJ, Lew GJ (1985) Distribution of actin in isolated seminiferous epithelia and denuded tubule walls of the rat. Anat Rec 213:63–71
Yazama F, Esaki M, Sawada H (1997) Immunocytochemistry of extracellular matrix components in the rat seminiferous tubule: electron microscopic localization with improved methodology. Anat Rec 248:51–62
Acknowledgments
This study was supported by the following grants: 06 J391, J025 and 06 J442 from SECyTP, UNCuyo, Argentina. The authors thank Dr. Silvia A. Belmonte for her most helpful comments on this manuscript. We appreciate the skilled technical assistance of Ing. Norberto Domizio and Ing. Elisa Bocanegra with light and electron microscopy. The authors also thank Stephen Anderson for editing assistance with the manuscript.
Author contributions
A.D.L. contributed to the study design, acquisition and analysis of data for light and confocal microscopy and preparation of the manuscript. V.S. contributed to data analysis and interpretation for scanning electron microscopy. M.E. and F.E. contributed to perform the immunolocalizations. L.A.L contributed to drafting and critical revision of the manuscript. A.M contributed to study design, acquisition and analysis of data for light and transmission electron microscopy and preparation of the manuscript. All authors revised the manuscript and have approved the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Losinno, A.D., Sorrivas, V., Ezquer, M. et al. Changes of myoid and endothelial cells in the peritubular wall during contraction of the seminiferous tubule. Cell Tissue Res 365, 425–435 (2016). https://doi.org/10.1007/s00441-016-2386-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2386-x