Abstract
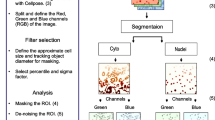
Comparing the distribution of cytoplasmic particles and organelles between different experimental conditions can be challenging due to the heterogeneous nature of cell morphologies. The border-to-border distribution method was created to enable the quantitative analysis of fluorescently labeled cytoplasmic particles and organelles of multiple cells from images obtained by confocal microscopy. The method consists of four steps: (1) imaging of fluorescently labeled cells, (2) division of the image of the cytoplasm into radial segments, (3) selection of segments of interest, and (4) population analysis of fluorescence intensities at the pixel level either as a function of distance along the selected radial segments or as a function of angle around an annulus. The method was validated using the well-characterized effect of brefeldin A (BFA) on the distribution of the vesicular stomatitis virus G protein, in which intensely labeled Golgi membranes are redistributed within the cytoplasm. Surprisingly, in untreated cells, the distribution of fluorescence in Golgi membrane-containing radial segments was similar to the distribution of fluorescence in other G protein-containing segments, indicating that the presence of Golgi membranes did not shift the distribution of G protein towards the nucleus compared to the distribution of G protein in other regions of the cell. Treatment with BFA caused only a slight shift in the distribution of the brightest G protein-containing segments which had a distribution similar to that in untreated cells. Instead, the major effect of BFA was to alter the annular distribution of G protein in the perinuclear region.







Similar content being viewed by others
Abbreviations
- BFA:
-
Brefeldin A
- BTBDM:
-
Border-to-border distribution method
- ER:
-
Endoplasmic reticulum
- MTOC:
-
Microtubule organizing center
- PNH:
-
Perinuclear high
- PNL:
-
Perinuclear low
- VSV:
-
Vesicular stomatitis virus
References
Bannykh SI, Plutner H, Matteson J, Balch WE (2005) The role of ARF1 and rab GTPases in polarization of the Golgi stack. Traffic 6(9):803–819. doi:10.1111/j.1600-0854.2005.00319.x
Barzilay E, Ben-Califa N, Hirschberg K, Neumann D (2005) Uncoupling of brefeldin a-mediated coatomer protein complex-I dissociation from Golgi redistribution. Traffic 6(9):794–802. doi:10.1111/j.1600-0854.2005.00317.x
Bergmann JE, Tokuyasu KT, Singer SJ (1981) Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A 78(3):1746–1750
Burkhardt JK, Hester S, Argon Y (1989) The glycoprotein of VSV accumulates in a distal Golgi compartment in the presence of CCCP. J Cell Sci 92(4):643–654
Doms RW, Russ G, Yewdell JW (1989) Brefeldin A redistributes resident and itinerant golgi proteins to the endoplasmic reticulum. J Cell Biol 109:61–72
Donaldson JG, Finazzi D, Klausner RD (1992) Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360:350–352
Goldenberg NM, Grinstein S, Silverman M (2007) Golgi-bound Rab34 is a novel member of the secretory pathway. Mol Biol Cell 18(12):4762–4771. doi:10.1091/mbc.E06-11-0991
Gue M, Messaoudi C, Sun JS, Boudier T (2005) Smart 3D-FISH: automation of distance analysis in nuclei of interphase cells by image processing. Cytom A 67(1):18–26. doi:10.1002/cyto.a.20170
Hamilton N (2009) Quantification and its applications in fluorescent microscopy imaging. Traffic 10(8):951–961. doi:10.1111/j.1600-0854.2009.00938.x
Helms JB, Rothman JE (1992) Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 360:352–354
Lippincott-Schwartz J (1990) Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin a suggests an ER recycling pathway. Cell 60(5):821–836. doi:10.1016/0092-8674(90)90096-W
Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR (2001) Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci U S A 98(5):2399–2406
Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y (1986) Novel blockade by brefeldin a of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem 261(24):11398–11403
Nebenfuhr A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130:1102–1108
Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL (1999) Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell 3:275–285
Rao M, Mayor S (2014) Active organization of membrane constituents in living cells. Curr Opin Cell Biol 29:126–132
Rasband WS (1997–2015) Image J. U.S. National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/
R Core Team (2015) R: a language environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Rogalski AA, Bergmann JE, Singer SJ (1984) Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J Cell Biol 99(3):1101–1109
Sutterlin C, Colanzi A (2010) The Golgi and the centrosome: building a functional partnership. J Cell Biol 188(6):621–628
Takatsuki A, Tamura G (1985) Brefeldin A, a specific inhibitor of intracellular translocation of vesicular stomatitis virus G protein: intracellular accumulation of high-mannose type G protein and inhibition of its cell surface expression. Agric Biol Chem 49(3):899–902
Terasaki M, Reese TS (1994) Interactions among endoplasmic reticulum, microtubules, and retrograde movements of the cell surface. Cell Motil Cytoskeleton 29:291–300
Vermolen BJ, Garini Y, Young IT, Dirks RW, Raz V (2008) Segmentation and analysis of the three-dimensional redistribution of nuclear components in human mesenchymal stem cells. Cytom A 73(9):816–824. doi:10.1002/cyto.a.20612
Waterman-Storer CM, Salmon ED (1998) Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol 8:798–806
Wozniak MJ, Allen VJ (2009) Carrier motility. In: Segev N (ed) Trafficking inside cells: pathways, mechanisms and regulation. Landes Bioscience, Austin, pp 233–253
Yadav S, Linstedt AD (2011) Golgi positioning. Cold spring harb. Perspect Biol 3:a005322
Zhu X, Kaverina I (2013) Golgi as an MTOC: making microtubules for its own good. Histochem Cell Biol 140:361–367
Acknowledgments
This study was supported by grants from the United States National Institute of Allergy and Infectious Diseases R01 AI015892 and R01 AI105012 (DSL) and National Cancer Institute RO1 CA127621 (DAO). We also acknowledge the support of the Cellular Imaging Shared Resource and the Cell and Virus Vector Core Laboratory of the Comprehensive Cancer Center of Wake Forest University supported by NCI grant P30 CA012197.404
Conflicts of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yacovone, S.K., Ornelles, D.A. & Lyles, D.S. The border-to-border distribution method for analysis of cytoplasmic particles and organelles. Cell Tissue Res 363, 351–360 (2016). https://doi.org/10.1007/s00441-015-2265-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2265-x