Abstract
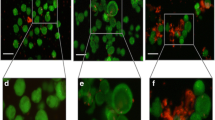
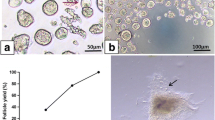
The risk of reintroducing malignant cells after ovarian graft into patients following post-cancer treatment is an obstacle for clinical applications (autotransplantation). In this context, in vitro follicle culture would be an alternative to transplantation in order to minimize such risks. Therefore, the aim of this study was to compare the development of secondary follicles after vitrification in isolated form (without stroma) with vitrification in in situ form (within fragments of ovarian tissue). Follicles were first isolated from ovarian fragments from mixed-breed ewes and then vitrified; these comprised the Follicle-Vitrification group (Follicle-Vit), or fragments of ovarian tissue were first vitrified, followed by isolation of the follicles, resulting in the Tissue-Vitrification group (Tissue-Vit). Control and vitrified groups were submitted to in vitro culture (6 days) and follicular morphology, viability, antrum formation, follicle and oocyte diameter, growth rate, ultrastructural characteristics and cell proliferation were evaluated. The percentages of morphologically normal follicles and antrum formation were similar among groups. Follicular viability and oocyte diameter were similar between Follicle-Vit and Tissue-Vit. The follicular diameter and growth rate of Follicle-Vit were similar to the Control, while those of Tissue-Vit were significantly lower compared to the Control. Both vitrified groups had an augmented rate of granulosa cellular proliferation compared to Control. Secondary follicles can be successfully vitrified before or after isolation from the ovarian tissue without impairing their ability to survive and grow during in vitro culture.





Similar content being viewed by others
References
Bian J, Li T, Ding C, Xin W, Zhu B, Zhou C (2013) Vitreous cryopreservation of human preantral follicles encapsulated in alginate beads with mini mesh cups. J Reprod Dev 59:288–295
Bordes A, Lornage J, Demirci B, Franck M, Courbiere B, Guerin JF, Salle B (2005) Normal gestations and live births after orthotopic autograft of vitrified-warmed hemi-ovaries into ewes. Hum Reprod 20:2745–2748
Castro SV, Carvalho AA, Gomes Silva CM, Santos FW, Campello CC, de Figueiredo JR, Ribeiro Rodrigues AP (2014) Frozen and fresh ovarian tissue require different culture media to promote in vitro development of bovine preantral follicles. Biopreserv Biobank 12:317–324
Cecconi S, Capacchietti G, Russo V, Berardinelli P, Mattioli M, Barboni B (2004) In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biol Reprod 70:12–17
Dolmans M-M, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J (2010) Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 116:2908–2914
Donnez J, Dolmans M-M (2013) Fertility preservation in women. Nat Rev Endocrinol 9:735–749
Duarte ABG, Araujo VR, Chaves RN, Silva GM, Magalhaes-Padilha DM, Satrapa RA, Donato MAM, Peixoto CA, Campello CC, Matos MHT, Barros CM, Figueiredo JR (2012) Bovine dominant follicular fluid promotes the in vitro development of goat preantral follicles. Reprod Fertil Dev 24:490–500
Fuku E, Xia L, Downey BR (1995) Ultrastructural-changes in bovine oocytes cryopreserved by vitrification. Cryobiology 32:139–156
Gosden RG, Baird DT, Wade JC, Webb R (1994) Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196-degrees-C. Hum Reprod 9:597–603
Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J (2006) Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril 85:1208–1215
Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, Braun F, Sadek F, van der Ven H (2006) Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reprod Biomed Online 13:228–234
Isachenko V, Isachenko E, Weiss JM, Todorov P, Kreienberg R (2009) Cryobanking of human ovarian tissue for anti-cancer treatment: comparison of vitrification and conventional freezing. Cryo Letters 30:449–454
Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K (2006) American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 24:2917–2931
Lin TC, Yen JM, Kuo TC, Gong KB, Hsu KH, Hsu TT (2008) Comparison of the developmental potential of 2-week-old preantral follicles derived from vitrified ovarian tissue slices, vitrified whole ovaries and vitrified/transplanted newborn mouse ovaries using the metal surface method. BMC Biotechnol 8:13
Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P (2006) Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol 299:1–11
Liu LJ, Xie XY, Zhang RZ, Xu P, Bujard H, Jun M (2008) Reproduction and fertility in wild-type and transgenic mice after orthotopic transplantation of cryopreserved ovaries from 10-d-old mice. Lab Anim 37:353–357
Lornage J, Courbière B, Mazoyer C, Odagescu V, Baudot A, Bordes A, Poirel MT, Franck M, Salle B (2006) Ovarian tissue vitrification: cortex and whole ovary in sheep. Gynecol Obstet Fertil 34:746–753
Lucci CM, Silva RV, Carvalho CA, Figueiredo R, Bao N (2001) Light microscopical and ultrastructural characterization of goat preantral follicles. Small Rumin Res 41:61–69
Lunardi FO, Araujo VR, Faustino LR, Carvalho AA, Braga Goncalves RF, Bass CS, Bao SN, Olazia Name KP, Campello CC, de Figueiredo JR, Ribeiro Rodrigues AP (2012) Morphologic, viability and ultrastructural analysis of vitrified sheep preantral follicles enclosed in ovarian tissue. Small Rumin Res 107:121–130
Luz VB, Araujo VR, Duarte ABG, Celestino JJH, Silva TFP, Magalhaes-Padilha DM, Chaves RN, Brito IR, Almeida AP, Campello CC, Feltrin C, Bertolini M, Santos RR, Figueiredo JR (2012) Eight-cell parthenotes originated from in vitro grown sheep preantral follicles. Reprod Sci 19:1219–1225
Luz VB, Araujo VR, Duarte ABG, Silva GM, Chaves RN, Brito IR, Serafim MKB, Campello CC, Feltrin C, Bertolini M, Almeida AP, Santos RR, Figueiredo JR (2013) Kit ligand and insulin-like growth factor I affect the in vitro development of ovine preantral follicles. Small Rumin Res 115:99–102
Nagano M, Atabay EP, Atabay EC, Hishinuma M, Katagiri S, Takahashi Y (2007) Effects of isolation method and pre-treatment with ethylene glycol or raffinose before vitrification on in vitro viability of mouse preantral follicles. Biomed Res 28:153–160
Oktay K, Karlikaya GG, Aydin BA (2000) Ovarian cryopreservation and transplantation: basic aspects. Mol Cell Endocrinol 169:105–108
Oktem O, Alper E, Balaban B, Palaoglu E, Peker K, Karakaya C, Urman B (2011) Vitrified human ovaries have fewer primordial follicles and produce less antimullerian hormone than slow-frozen ovaries. Fertil Steril 95:2661
Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E (2011) Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod 26:1097–1103
Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J (2002) Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril 77:403–408
Salle B, Demirci B, Franck M, Berthollet C, Lornage J (2003) Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: pregnancies, births, and histologic assessment. Fertil Steril 80:172–177
Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, Paris M, Schoenfeldt V, Martinez-Madrid B (2010) Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci 122:151–163
Silva CM, Serakides R, Nascimento EF, Nunes VA, Ribeiro AFC, Ocarino NM (2003) Quantification of the nucleolar organizer regions (NORs) as a parameter to measure proliferation of granulosa cells. Arq Bras Med Vet Zootec 55:113–116
Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U (2010) DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Hum Reprod 25:3025–3042
Van den Abbeel E, Schneider U, Liu J, Agca Y, Critser JK, Van Steirteghem A (2007) Osmotic responses and tolerance limits to changes in external osmolalities, and oolemma permeability characteristics, of human in vitro matured MII oocytes. Hum Reprod 22:1959–1972
Vanacker J, Luyckx V, Amorim C, Dolmans M-M, Van Langendonckt A, Donnez J, Camboni A (2013) Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril 99:1363
Xing W, Zhou C, Bian J, Montag M, Xu Y, Li Y, Li T (2010) Solid-surface vitrification is an appropriate and convenient method for cryopreservation of isolated rat follicles. Reprod Biol Endocrinol 8:42–50
Financial support
This work was carried out at the Laboratory of Manipulation of Oocytes and Ovarian Preantral Follicles (LAMOFOPA), Faculty of Veterinary Medicine of Ceará State University, Fortaleza, CE, Brazil, and supported by CNPq. Franciele Osmarini Lunardi is a recipient of a grant from CAPES Brazil. In addition, Ana Paula Ribeiro Rodrigues and José Ricardo de Figueiredo are recipients of a grant from CNPq Brazil.
Conflict of interest
The authors declare that there is no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lunardi, F.O., Chaves, R.N., de Lima, L.F. et al. Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture. Cell Tissue Res 362, 241–251 (2015). https://doi.org/10.1007/s00441-015-2181-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2181-0