Abstract
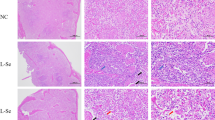
The purpose of the present study is to determine if visfatin is involved in inflammation or apoptosis induced by LPS in rat. Forty Wistar rats were divided into four groups: saline group, LPS group, visfatin group and Visfatin + LPS co-stimulated group. Spleen samples from each group of rats were collected for study. The spleen structure was examined by histological imaging. Apoptosis was evaluated with TUNEL reaction. Caspase-3 was detected with immunohistochemistry and western blot. The apoptosis-related genes were detected by qPCR and inflammatory cytokines were tested by ELISA. Our main findings were as follows. (1) Macrophages were markedly increased in the visfatin group compared with the saline group. This finding was confirmed when spleen samples were examined with western blot using CD68 antibody. (2) Visfatin promoted the expression of CD68 and caspase-3 in rat spleen, whereas visfatin could inhibit the expression of CD68 and activated caspase-3 in spleen of LPS-induced acute inflammation. (3) Visfatin had a pro-apoptotic effect on normal rat spleen, whereas it exerted an anti-apoptotic effect during LPS-induced lymphocytes apoptosis in rat spleen. Moreover, the effect of visfatin on cell apoptosis was mediated by the mitochondrial pathway. (4) Visfatin could modulate both the anti-inflammatory cytokines and pro-inflammatory cytokines in rat spleen, such as IL-10, IL-4, IL-6, TNF-α and IL-1β. Taken together, we demonstrate that visfatin could participate in the inflammatory process in rat spleen by modulating the macrophages and inflammatory cytokines. Also, visfatin plays a dual role in the apoptosis in rat spleen, which is mediated by the mitochondrial pathway.









Similar content being viewed by others
Abbreviations
- bw:
-
Body weight
- Casp-3:
-
Caspase-3
- HE staining:
-
Hematoxylin and Eosin staining
- IL-1β:
-
Interleukin-1β
- IL-4:
-
Interleukin-4
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IOD:
-
Integral optical density
- LPS:
-
Lipopolysaccharide
- MD:
-
Mean density
- PALS:
-
Periarterial lymphatic sheath
- PBEF:
-
Pre-B cell colony-enhancing factor
- TNF-α:
-
Tumor necrosis factor-α
- TUNEL:
-
Terminal-deoxynucleoitidyl transferase mediated nick end labeling
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng A 14:1835–1842
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Barrett KL, Willingham JM, Garvin AJ, Willingham MC (2001) Advances in cytochemical methods for detection of apoptosis. J Histochem Cytochem 49:821–832
Cheng Q, Dong W, Qian L, Wu J, Peng Y (2011) Visfatin inhibits apoptosis of pancreatic β-cell line, MIN6, via the mitogen-activated protein kinase/phosphoinositide 3-kinase pathway. J Mol Endocrinol 47:13–21
Curat C, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A (2006) Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49:744–747
de Fougerolles AR, Chi-Rosso G, Bajardi A, Gotwals P, Green CD, Koteliansky VE (2000) Global expression analysis of extracellular matrix–integrin interactions in monocytes. Immunity 13:749–758
Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–2147
Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA (2003) Comparison of immunohistochemistry for activated caspase‐3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC‐3 subcutaneous xenografts. J Pathol 199:221–228
Dukers DF, Oudejans JJ, Vos W, ten Berge RL, Meijer CJ (2002) Apoptosis in B‐cell lymphomas and reactive lymphoid tissues always involves activation of caspase 3 as determined by a new in situ detection method. J Pathol 196:307–315
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A (2001) Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol 2:316–324
Fuller KA, Kanagawa O, Nahm MH (1993) T cells within germinal centers are specific for the immunizing antigen. J Immunol 151:4505–4512
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Hall P (1999) Assessing apoptosis: a critical survey. Endocr Relat Cancer 6:3–8
Hanada M, Aime-Sempe C, Sato T, Reed JC (1995) Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem 270:11962–11969
Holness CL, Da Silva R, Fawcett J, Gordon S, Simmons D (1993) Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem 268:9661–9666
Hongmei Z (2012) Extrinsic and intrinsic apoptosis signal pathway review. Apoptosis and Medicine, InTech
Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC (2004) Pre–B cell colony–enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Investig 113:1318–1327
Jiang X, Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73:87–106
Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A (2008) Predominant infiltration of macrophages and CD8+ T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113:1387–1395
Kelsoe G (1996) The germinal center: a crucible for lymphocyte selection. Semin Immunol 8:179–184
Kim D, Min H, Lee K, Kim Y, Oh D, Jeon Y, Lee S, Im S, Chung D, Kim Y (2008) High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer 98:1118–1124
Koczan D, Guthke R, Thiesen H-J, Ibrahim SM, Kundt G, Krentz H, Gross G, Kunz M (2005) Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur J Dermatol 15:251–257
Korsmeyer SJ (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59:1693s–1700s
Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M (2005a) Hormonal regulation of the novel adipocytokine visfatin in 3 T3-L1 adipocytes. J Endocrinol 185:R1–R8
Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M (2005b) Interleukin-6 is a negative regulator of visfatin gene expression in 3 T3-L1 adipocytes. Am J Physiol Endocrinol Metab 289:E586–E590
Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 3:614–620
Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, S-i I, Tabas I (2008) Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem 283:34833–34843
Liu K, Li Y, Prabhu V, Young L, Becker KG, Munson PJ, Weng N-p (2001) Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. J Immunol 166:7335–7344
Luk T, Malam Z, Marshall JC (2008) Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol 83:804–816
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112
MacLennan IC (1994) Germinal centers. Annu Rev Immunol 12:117–139
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13:265–270
Mantovani A, Sica A, Allavena P, Garlanda C, Locati M (2009) Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol 70:325–330
Mayi TH, Duhem C, Copin C, Bouhlel MA, Rigamonti E, Pattou F, Staels B, Chinetti-Gbaguidi G (2010) Visfatin is induced by peroxisome proliferator‐activated receptor gamma in human macrophages. FEBS J 277:3308–3320
McGlothlin JR, Gao L, Lavoie T, Simon BA, Easley RB, Ma S-F, Rumala BB, Garcia JG, Ye SQ (2005) Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem Genet 43:127–141
Mebius RE, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5:606–616
Moore KJ, Freeman MW (2006) Scavenger receptors in atherosclerosis beyond lipid uptake. Arterioscler Thromb Vasc Biol 26:1702–1711
Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H (2007) Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 178:1748–1758
Moschen AR, Gerner RR, Tilg H (2010) Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr Pharm Des 16:1913–1920
Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA (2002) Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci USA 99:1503–1508
Newburger PE, Subrahmanyam Y, Weissman SM (2000) Global analysis of neutrophil gene expression. Curr Opin Hematol 7:16–20
Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, Bryant-Greenwood G, Jones SA (2006) Regulation of pre–B cell colony‐enhancing factor by STAT‐3–dependent interleukin‐6 trans‐signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 54:2084–2095
Ognjanovic S, Bryant-Greenwood GD (2002) Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol 187:1051–1058
Ognjanovic S, Ku TL, Bryant-Greenwood GD (2005) Pre–B-cell colony–enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am J Obstet Gynecol 193:273–282
Ohri C, Shikotra A, Green R, Waller D, Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33:118–126
Oki K, Yamane K, Kamei N, Nojima H, Kohno N (2007) Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol 67:796–800
Peters M, Jacobs S, Ehlers M, Vollmer P, Müllberg J, Wolf E, Brem G, zum Büschenfelde KM, Rose-John S (1996) The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med 183:1399–1406
Rathmell JC, Thompson CB (2002) Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109:S97–S107
Resendes AR, Majó N, Segalés J, Mateu E, Calsamiglia M, Domingo M (2004) Apoptosis in lymphoid organs of pigs naturally infected by porcine circovirus type 2. J Gen Virol 85:2837–2844
Romagnolo DF, Davis CD, Milner JA (2012) Phytoalexins in cancer prevention. Front Biosci 17:2035–2058
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F (2002) Pre‐B‐cell colony‐enhancing factor, whose expression is up‐regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol 32:3225–3234
Rongvaux A, Galli M, Denanglaire S, Van Gool F, Drèze PL, Szpirer C, Bureau F, Andris F, Leo O (2008) Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol 181:4685–4695
Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H (1993) Mice lacking the tumour necrosis factor receptor 1 are resistant to IMF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798–802
Rudel T (1999) Caspase inhibitors in prevention of apoptosis. Herz 24:236–241
Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91:443–446
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 14:1431–1437
Samara A, Pfister M, Marie B, Visvikis-Siest S (2008) Visfatin, low‐grade inflammation and body mass index (BMI). Clin Endocrinol 69:568–574
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A 92:7834–7838
Seo JA, Jang ES, Kim BG, Ryu OH, Kim HY, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS (2008) Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women. Diabetes Res Clin Pract 79:108–111
Shaffer A, Rosenwald A, Hurt EM, Giltnane JM, Lam LT, Pickeral OK, Staudt LM (2001) Signatures of the immune response. Immunity 15:375–385
Shalhoub J, Falck-Hansen MA, Davies AH, Monaco C (2011) Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm (Lond) 8:9
Spinozzi F, de Benedictis D, de Benedictis FM (2008) Apoptosis, airway inflammation and anti-asthma therapy: from immunobiology to clinical application. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 19:287–295
Stadelmann C, Lassmann H (2000) Detection of apoptosis in tissue sections. Cell Tissue Res 301:19–31
Teague TK, Hildeman D, Kedl RM, Mitchell T, Rees W, Schaefer BC, Bender J, Kappler J, Marrack P (1999) Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci USA 96:12691–12696
Tough DF, Sun S, Sprent J (1997) T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 185:2089–2094
Traves PG, Luque A, Hortelano S (2012) Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediat Inflamm 2012:568783
van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG (2005) Pre–B-cell colony–enhancing factor regulates NAD + −dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res 97:25–34
Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P (2006) Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211:487–501
Wang P, Xu T-Y, Guan Y-F, Su D-F, Fan G-R, Miao C-Y (2009) Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 81:370–380
Willingham MC (1999) Cytochemical methods for the detection of apoptosis. J Histochem Cytochem 47:1101–1109
Xiao J, Sun B, Li M, Wu Y, Sun XB (2013) A novel adipocytokine visfatin protects against H2O2‐induced myocardial apoptosis: a missing link between obesity and cardiovascular disease. J Cell Physiol 228:495–501
Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, Easley RB, McVerry BJ, Tuder RM, Standiford T (2005) Pre–B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 171:361–370
Zhan Y, van de Water B, Wang Y, Stevens JL (1999) The roles of caspase-3 and bcl-2 in chemically-induced apoptosis but not necrosis of renal epithelial cells. Oncogene 18:6505
Zheng B, Han S, Kelsoe G (1996) T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J Exp Med 184:1083–1091
Acknowledgments
We acknowledge grant support from the “Fundamental Research Funds for the Central Universities” (№. 2012SC02). This study was also supported by a grant from the National Natural Science Foundation of China under grant agreement (№.31101776). We gratefully acknowledge the expert advice of Mr. Juming Zhong and thank him for his detailed review of the manuscript; College of Veterinary Medicine, Auburn University, USA.
Competing interests
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, K., Zou, WH., Yang, Z. et al. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res 359, 605–618 (2015). https://doi.org/10.1007/s00441-014-1997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1997-3