Abstract
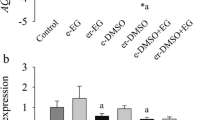
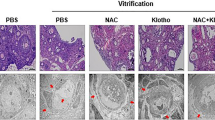
Ovarian fragments were exposed to 0.5 M sucrose and 1 M ethylene glycol (freezing solution; FS) with or without selenium or Trolox. Histological and ultrastructural analyses showed that the percentages of normal follicles in control tissue and in tissue after exposure to FS + 50 μM Trolox were similar. Trolox prevented endoplasmic reticulum (ER)-related vacuolization, which is commonly observed in oocytes and stromal tissue after exposure to FS. From the evaluated stress markers, superoxide dismutase 1 (SOD1) was up-regulated in ovarian tissue exposed to FS + 10 ng/ml selenium. Ovarian fragments were subsequently frozen-thawed in the presence of FS with or without 50 μM Trolox, followed by in vitro culture (IVC). Antioxidant capacity in ovarian fragments decreased after freeze-thawing in Trolox-free FS compared with FS + 50 μM Trolox. Although freezing itself minimized the percentage of viable follicles in each solution, Trolox supplementation resulted in higher rates of viable follicles (67 %), even after IVC (61 %). Furthermore, stress markers SOD1 and ERp29 were up-regulated in ovarian tissue frozen-thawed in Trolox-free medium. Relative mRNA expression of growth factors markers was evaluated after freeze-thawing followed by IVC. BMP4, BMP5, CTGF, GDF9 and KL were down-regulated independently of the presence of Trolox in FS but down-regulation was less pronounced in the presence of Trolox. Thus, medium supplementation with 50 μM Trolox prevents ER stress and, consequently, protects ovarian tissue from ER-derived cytoplasmic vacuolization. ERp29 but not ERp60, appears to be a key marker linking stress caused by freezing-thawing and cell vacuolization.




Similar content being viewed by others
References
Amorim CA, Jacobs S, Devireddy RV, Van Langendonckt A, Vanacker J, Jaeger J, Luyckx V, Donnez J, Dolmans MM (2013) Successful vitrification and autografting of baboon (Papio Anubis) ovarian tissue. Hum Reprod 28:2146–2156
Banjerdpongchai R, Kongtawelert B, Khantamat O, Srisomsap C, Chokcahichamnankit D, Subhasitanont P, Svasti J (2010) Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J Hematol Oncol 3:50
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach for multiple testing. J R Stat Soc Ser B 57:289–300
Bibi A, Agarwal NK, Dihazi GH, Eltoweissy M, Van Nguyen P, Mueller GA, Dihazi H (2011) Calreticulin is crucial for calcium homeostasis mediated adaptation and survival of thick ascending limb of Henle's loop cells under osmotic stress. Int J Biochem Cell Biol 43:1187–1197
Boonkusol D, Gal AB, Bodo S, Gorhony B, Kitiyanant Y, Dinnyes A (2006) Gene expression profiles and in vitro development following vitrification of pronuclear and 8-cell stage mouse embryos. Mol Reprod Dev 73:700–708
Bradford A, Atkinson J, Fuller N, Rand RP (2003) The effect of vitamin E on the structure of membrane lipid assemblies. J Lipid Res 44:1940–1945
Brito AB, Santos RR, Van den Hurk R, Lima JS, Miranda MS, Ohashi OM, Domingues SFS (2013a) Short-term culture of ovarian cortical strips from capuchin monkeys (Sapajus apella): a morphological, viability, and molecular study of preantral follicular development in vitro. Reprod Sci 20:990–997
Brito AB, Lima JS, Brito DC, Santana LN, Costa NN, Miranda MS, Ohashi OM, Santos RR, Domingues SFS (2013b) Validation of reference genes for ovarian tissue from capuchin monkeys (Cebus apella). Zygote 21:167–171
Celestino JJH, Santos RR, Lopes CAP, Martins FS, Matos MHT, Melo MAP, Báo SN, Rodrigues APR, Silva JRV, Figueiredo JR (2008) Preservation of bovine preantral follicle viability and ultra-structure after cooling and freezing of ovarian tissue. Anim Reprod Sci 108:309–318
Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, Macchiarelli G, Nottola SA (2010) Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Genet 27:131–140
Dinara S, Sengoku K, Tamate K, Horikawa M, Ishikawa M (2001) Effects of supplementation with free radical scavengers on the survival and fertilization rates of mouse cryopreserved oocytes. Hum Reprod 16:1976–1981
Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola AS, Donnez J (2006) Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod 21:413–420
Domingues SFS, Ferreira HS, Muniz JAPC, Lima AKF, Ohashi OM, Figueiredo JR, Silva LDM (2003) Mechanical isolation of capuchin monkey (Cebus apella) PF. Arq Bras Med Vet Zootec 55:301–308
Fahy GM (2010) Cryoprotectant toxicity neutralization. Cryobiology 60:45–53
Gook DA, Edgar DH, Stern C (2000) The effects of cryopreservation regimens on the morphology of human ovarian tissue. Mol Cell Endocrinol 27:99–103
Hubbard MJ, McHugh NJ, Carne DL (2000) Isolation of ERp29, a novel endoplasmic reticulum protein, from rat enamel cells. Evidence for a unique role in secretory-protein synthesis. Eur J Biochem 267:1945–1957
Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, Gosden RG (2004) Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril 82:679–687
Larman MG, Sheehan CB, Gardner DK (2006) Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction 131:53–61
Lopez-Martinez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE Jr, Denlinger DL (2009) Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. J Comp Physiol [B] 179:481–491
Lowther KM, Weitzman VN, Maier D, Mehlmann LM (2009) Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol Reprod 81:147–154
Luz HKM, Santos RR, Wanderley LS, Faustino LR, Silva CMG, Carvalho AA, Campello CC, Santos FW, Figueiredo JR, Rodrigues APR (2012) Catalase prevents lipid peroxidation and enhances survival of caprine preantral follicles cryopreserved in a 1,2-propanediol-freezing medium. Biopreserv Biobank 10:338–342
Marsella T, Sena P, Xella S, La Marca A, Giulini S, De Pol A, Volpe A, Marzona L (2008) Human ovarian tissue cryopreservation: effect of sucrose concentration on morphological features after thawing. Reprod Biomed Online 16:257–267
Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM (1998) Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139:4008–4011
Melo MA, Oskam IC, Celestino JJ, Carvalho AA, Castro SV, Figueiredo JR, Rodrigues AP, Santos RR (2011) Adding ascorbic acid to vitrification and IVC medium influences preantral follicle morphology, but not viability. Reprod Domest Anim 46:742–745
Nilsson EE, Skinner MK (2003) Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 69:1265–1272
Nottola SA, Coticchio G, De Santis L, Macchiarelli G, Maione M, Bianchi S, Iaccarino M, Flamigni C, Borini A (2008) Ultrastructure of human mature oocytes after slow cooling cryopreservation with ethylene glycol. Reprod Biomed Online 17:368–377
Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, Bianchi S, Macchiarelli G, Borini A (2009) Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online 19:17–27
Oskam IC, Asadi BA, Santos RR (2010) Histologic and ultrastructural features of cryopreserved ovine ovarian tissue: deleterious effect of 1,2-propanediol applying different thawing protocols. Fertil Steril 93:2764–2766
Oskam I, Lund T, Santos R (2011) Irreversible damage in ovine ovarian tissue after cryopreservation in propanediol: analyses after in vitro culture and xenotransplantation. Reprod Domest Anim 46:793–799
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Re R, Pellegrini R, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, Van den Hoff MJ et al (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative qPCR. Nucleic Acids Res 37:e45
Sanfilippo S, Canis M, Romero S, Sion B, Déchelotte P, Pouly J-L, Janny L, Smitz J, Brugnon F (2013) Quality and functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J Assist Reprod Genet 30:25–34
Santana LN, Brito AB, Brito DC, Lima JS, Domingues SFS, Santos RR (2013) Adaptation of a trap door technique for the recovery of ovarian cortical biopsies from Cebus apella (capuchin monkey). Zygote 21:158–161
Santos RR, Tharasanit T, Figueiredo JR, Van Haeften T, Van den Hurk R (2006a) Preservation of caprine preantral follicle viability after cryopreservation in sucrose and ethylene glycol. Cell Tissue Res 325:523–531
Santos RR, Rodrigues AP, Costa SH, Silva JR, Matos MH, Lucci CM, Báo SN, Van den Hurk R, Figueiredo JR (2006b) Histological and ultrastructural analysis of cryopreserved sheep PF. Anim Reprod Sci 91:249–263
Santos RR, Amorim CA, Cecconi S, Fassbender M, Imhof M, Lornage J, Paris M, Schoenfeldt V, Martinez-Madrid B (2010) Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci 122:151–163
Sheikhi M, Hultenby K, Niklasson B, Lundgvist M, Hovatta O (2013) Preservation of human ovarian follicles within tissue frozen by vitrification in a xeno-free closed system using only ethylene glycol as a permeating cryoprotectant. Fertil Steril 100:171–177
Silva JR, Lucci CM, Carvalho FC, Báo SN, Costa SH, Santos RR, Figueiredo JR (2000) Effect of coconut water and Braun-Collins solutions at different temperatures and incubation times on the morphology of goat PF preserved in vitro. Theriogenology 54:809–822
Spindler R, Wolkers WF, Glasmacher B (2011) Dimethyl sulfoxide and ethylene glycol promote membrane phase change during cryopreservation. Cryo Letters 32:148–157
Ting AY, Yeoman RR, Lawson MS, Zelinski MB (2011) In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod 26:2461–2472
Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB (2013) Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod 28:1267–1279
Turathum B, Saikhun K, Sangsuwan P, Kitiyanant Y (2010) Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod Biol Endocrinol 8:70
Uğuz AC, Naziroğlu M, Espino J, Bejarano I, González D, Rodríguez AB, Pariente JA (2009) Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and -9 activities. J Membr Biol 232:15–23
Van den Hurk R, Zhao J (2005) Formation of mammalian oocytes and their growth differentiation and maturation within ovarian follicles. Theriogenology 63:1717–1751
Younis AI, Rooks B, Khan S, Gould KG (1998) The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J Androl 19:207–214
Zhang D, Richardson DR (2011) Endoplasmic reticulum protein 29 (ERp29): an emerging role in cancer. Int J Biochem Cell Biol 43:33–36
Acknowledgments
The authors thank CENP for logistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by project no. 483439/2009-6 from CNPq, Brazil.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 34 kb)
Supplementary Table 2
(DOC 34 kb)
Rights and permissions
About this article
Cite this article
Brito, D.C., Brito, A.B., Scalercio, S.R.R.A. et al. Vitamin E-analog Trolox prevents endoplasmic reticulum stress in frozen-thawed ovarian tissue of capuchin monkey (Sapajus apella). Cell Tissue Res 355, 471–480 (2014). https://doi.org/10.1007/s00441-013-1764-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1764-x