Abstract
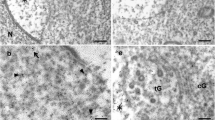
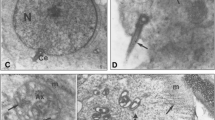
Spermatids must precisely integrate specific molecules into structurally supported domains that develop during spermatogenesis. Once established, the architecture of the acrosome contributes to the acrosome reaction, which occurs prior to gamete interaction in mammals. The present study aims to clarify the morphology associated with the integration of the mouse fertilization-related acrosomal protein equatorin (mEQT) into the developing acrosome. EQT mRNA was first detected by in situ hybridization in round spermatids but disappeared in early elongating spermatids. The molecular size of mEQT was approximately 65 kDa in the testis. Developmentally, EQT protein was first detected on the nascent acrosomal membrane in round spermatids at approximately step 3, was actively integrated into the acrosomal membranes of round spermatids in the following step and then participated in acrosome remodeling in elongating spermatids. This process was clearly visualized by high-resolution fluorescence microscopy and super-resolution stimulated emission depletion nanoscopy by using newly generated C-terminally green-fluorescent-protein-tagged mEQT transgenic mice. Immunogold electron microscopy revealed that mEQT was anchored to the acrosomal membrane, with the epitope region observed as lying 5–70 nm away from the membrane and was associated with the electron-dense acrosomal matrix. This new information about the process of mEQT integration into the acrosome during spermatogenesis should provide a better understanding of the mechanisms underlying not only acrosome biogenesis but also fertilization and male infertility.








Similar content being viewed by others
References
Anderson DJ, Michaelson JS, Johnson PM (1989) Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol Reprod 41:285–293
Bennett EP, Hassan H, Clausen H (1996) cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3. J Biol Chem 271:17006–17012
Bi M, Hickox JR, Winfrey VP, Olson GE, Hardy DM (2003) Processing, localization, and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem J 375:477–488
Eddy EM (2006) The spermatozoon: gametes, fertilization, and embryogenesis. In: Wassarman PM (ed) Knobil and Neill’s physiology of reproduction, vol 1, 3rd edn. Elsevier/Academic Press, London, pp 3–54
Ferrer M, Rodriguez H, Zara L, Yu Y, Xu W, Oko R (2012) MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res 349:881–895
Foster JA, Friday BB, Maulit MT, Blobel C, Winfrey VP, Olson GE, Kim KS, Gerton GL (1997) AM67, a secretory component of the guinea pig sperm acrosomal matrix, is related to mouse sperm protein sp56 and the complement component 4-binding proteins. J Biol Chem 272:12714–12722
Fujihara Y, Satouh Y, Inoue N, Isotani A, Ikawa M, Okabe M (2012) SPACA1-deficient male mice are infertile with abnormally shaped sperm heads reminiscent of globozoospermia. Development 139:3583–3589
Hao Z, Wolkowicz MJ, Shetty J, Klotz K, Bolling L, Sen B, Westbrook VA, Coonrod S, Flickinger CJ, Herr JC (2002) SAMP32, a testis-specific, isoantigenic sperm acrosomal membrane-associated protein. Biol Reprod 66:735–744
Hell SW (2007) Far-field optical nanoscopy. Science 316:1153–1158
Hell SW, Wichmann J (1994) Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett 19:780–782
Herr JC, Flickinger CJ, Homyk M, Klotz K, John E (1990) Biochemical and morphological characterization of the intra-acrosomal antigen SP-10 from human sperm. Biol Reprod 42:181–193
Inoue N, Ikawa M, Isotani A, Okabe M (2005) The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434:234–238
Ito C, Yamatoya K, Yoshida K, Kyono K, Yao R, Noda T, Toshimori K (2010a) Appearance of an oocyte activation-related substance during spermatogenesis in mice and humans. Hum Reprod 25:2734–2744
Ito C, Yamatoya K, Yoshida K, Maekawa M, Miyado K, Toshimori K (2010b) Tetraspanin family protein CD9 in the mouse sperm: unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res 340:583–594
Kleene KC (1996) Patterns of translational regulation in the mammalian testis. Mol Reprod Dev 43:268–281
Kurohmaru M, Kanai Y, Hayashi Y (1991) Lectin-binding patterns in the spermatogenic cells of the shiba goat testis. J Vet Med Sci 53:893–897
Kurth BE, Klotz K, Flickinger CJ, Herr JC (1991) Localization of sperm antigen SP-10 during the six stages of the cycle of the seminiferous epithelium in man. Biol Reprod 44:814–821
Manandhar G, Toshimori K (2001) Exposure of sperm head equatorin after acrosome reaction and its fate after fertilization in mice. Biol Reprod 65:1425–1436
Miyazaki T, Mori M, Yoshida CA, Ito C, Yamatoya K, Moriishi T, Kawai Y, Komori H, Kawane T, Izumi S-I, Toshimori K, Komori T (2013) Galnt3 deficiency disrupts acrosome formation and leads to oligoasthenoteratozoospermia. Histochem Cell Biol 139:339–354
Mortimer D, Curtis EF, Miller RG (1987) Specific labelling by peanut agglutinin of the outer acrosomal membrane of the human spermatozoon. J Reprod Fertil 81:127–135
Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, Mimura T (1988) Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol 13:211–219
Olson GE, Winfrey VP, Bi M, Hardy DM, NagDas SK (2004) Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis. Biol Reprod 71:1128–1134
Overstreet JW, Lin Y, Yudin AI, Meyers SA, Primakoff P, Myles DG, Katz DF, Vandevoort CA (1995) Location of the PH-20 protein on acrosome-intact and acrosome-reacted spermatozoa of cynomolgus macaques. Biol Reprod 52:105–114
Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED (1990) Histological and histopathological evaluation of the testis. 1st edn. Cache River Press, Clearwater, Fl, USA
Sabeur K, Cherr GN, Yudin AI, Primakoff P, Li MW, Overstreet JW (1997) The PH-20 protein in human spermatozoa. J Androl 18:151–158
Shetty J, Wolkowicz MJ, Digilio LC, Klotz KL, Jayes FL, Diekman AB, Westbrook VA, Farris EM, Hao Z, Coonrod SA, Flickinger CJ, Herr JC (2003) SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J Biol Chem 278:30506–30515
Steger K (1999) Transcriptional and translational regulation of gene expression in haploid spermatids. Anat Embryol (Berl) 199:471–487
Tanii I, Oh-oka T, Yoshinaga K, Toshimori K (2001) A mouse acrosomal cortical matrix protein, MC41, has ZP2-binding activity and forms a complex with a 75-kDa serine protease. Dev Biol 238:332–341
Toshimori K (2009) Dynamics of the mammalian sperm head: modifications and maturation events from spermatogenesis to egg activation. Adv Anat Embryol Cell Biol 204:5–94
Toshimori K (2011) Dynamics of the mammalian sperm membrane modification leading to fertilization: a cytological study. J Electron Microsc 60 (Suppl 1):31–42
Toshimori K, Tanii I, Araki S, Oura C (1992) Characterization of the antigen recognized by a monoclonal antibody MN9: unique transport pathway to the equatorial segment of sperm head during spermiogenesis. Cell Tissue Res 270:459–468
Toshimori K, Tanii I, Araki S (1995) Intra-acrosomal 155,000 dalton protein increases the antigenicity during mouse sperm maturation in the epididymis: a study using a monoclonal antibody MC101. Mol Reprod Dev 42:72–79
Toshimori K, Saxena DK, Tanii I, Yoshinaga K (1998) An MN9 antigenic molecule, equatorin, is required for successful sperm-oocyte fusion in mice. Biol Reprod 59:22–29
Toyoda Y, Yokoyama M, Hoshi T (1971) Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn J Anim Reprod 16:147–151
Watanabe D, Okabe M, Hamajima N, Morita T, Nishina Y, Nishimune Y (1995) Characterization of the testis-specific gene “calmegin” promoter sequence and its activity defined by transgenic mouse experiments. FEBS Lett 368:509–512
Westbrook-Case VA, Winfrey VP, Olson GE (1994) A domain-specific 50-kilodalton structural protein of the acrosomal matrix is processed and released during the acrosome reaction in the guinea pig. Biol Reprod 51:1–13
Westbrook-Case VA, Winfrey VP, Olson GE (1995) Sorting of the domain-specific acrosomal matrix protein AM50 during spermiogenesis in the guinea pig. Dev Biol 167:338–349
Wolkowicz MJ, Shetty J, Westbrook A, Klotz K, Jayes F, Mandal A, Flickinger CJ, Herr JC (2003) Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol Reprod 69:735–745
Yamatoya K, Yoshida K, Ito C, Maekawa M, Yanagida M, Takamori K, Ogawa H, Araki Y, Miyado K, Toyama Y, Toshimori K (2009) Equatorin: identification and characterization of the epitope of the MN9 antibody in the mouse. Biol Reprod 81:889–897
Yoshida K, Ito C, Yamatoya K, Maekawa M, Toyama Y, Suzuki-Toyota F, Toshimori K (2010) A model of the acrosome reaction progression via the acrosomal membrane-anchored protein equatorin. Reproduction 139:533–544
Yoshinaga K, Saxena DK, Oh-oka T, Tanii I, Toshimori K (2001) Inhibition of mouse fertilization in vivo by intra-oviductal injection of an anti-equatorin monoclonal antibody. Reproduction 122:649–655
Yu Y, Xu W, Yi YJ, Sutovsky P, Oko R (2006) The extracellular protein coat of the inner acrosomal membrane is involved in zona pellucida binding and penetration during fertilization: characterization of its most prominent polypeptide (IAM38). Dev Biol 290:32–43
Yu Y, Vanhorne J, Oko R (2009) The origin and assembly of a zona pellucida binding protein, IAM38, during spermiogenesis. Microsc Res Tech 72:558–565
Acknowledgment
This study was partly performed by using a nanoscopy TCS STED CW (Leica Microsystems, Tokyo). The authors are grateful to Professor Ikawa and Professor Okabe at the Research Institute for Microbial Diseases, Osaka University for kindly providing the pCM vector for the mouse Calmegin promotor and to Ms. K Kamimura and Mr. T Mutoh for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Japan Society for the Promotion of Science partially via grants to K.T. (22112504, 22390033, 24112706) and in part by grants to C.I. (24592441) and to K.Y. (23791809).
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ito, C., Yamatoya, K., Yoshida, K. et al. Integration of the mouse sperm fertilization-related protein equatorin into the acrosome during spermatogenesis as revealed by super-resolution and immunoelectron microscopy. Cell Tissue Res 352, 739–750 (2013). https://doi.org/10.1007/s00441-013-1605-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1605-y