Abstract
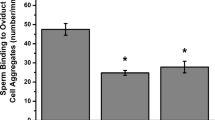
In mammals, interaction between sperm and oviductal epithelial cells provides the formation of a sperm reservoir and sperm selection at the isthmus of the oviduct. Several in vitro methods are used to study this interaction. Apical plasma membranes (APM) have been prepared by peeling from culture and differentiated kidney cells. In this work, we modify this method, using it for the preparation of APM directly from the whole oviduct, proving purity of the apical plasma membranes obtained by western blot for proteins of known specific locations. The obtained APM correspond only to the most differentiated cells, exposed at the lumen of the organ. Also, the prepared APM are shown by biotinylation to interact with sperm. The binding is at the head of sperm and induces on them prolonged motility and tyrosine phosphorylation of proteins of masses 92, 97, 210 and 220 kDa. The tyrosine phosphorylation of p97 has been previously described as an effect of the apical membrane exposed sperm binding glycoprotein (SBG), which is shown to be present in the preparations described here. Upon treatment with APM, the tyrosine phosphorylation pattern of sperm changes from heads to tail. Thus, we describe an easy method for APM preparation directly from organs that allows the study of oviductal proteins in their context and permits sperm–oviduct interaction studies. This method renders APM specifically from the cells located at the lumen of the oviduct.







Similar content being viewed by others
Bibliography
Althouse (1997) Evaluating porcine semen for artificial insemination: standard tests. Department of Veterinary Clinical Medicine, College of Veterinary Medicine, University of Illinois. Compend Contin Educ Pract Vet 19(Suppl): 30–35
Ardon F, Helms D, Sahin E, Bollwein H, Topfer-Petersen E, Waberski D (2008) Chromatin-unstable boar spermatozoa have little chance of reaching oocytes in vivo. Reproduction 135:461–470
Boilard M, Bailey J, Collin S, Dufour M, Sirard MA (2002) Effect of bovine oviduct epithelial cell apical plasma membranes on sperm function assessed by a novel flow cytometric approach. Biol Reprod 67:1125–1132
Bureau M, Bailey JL, Sirard MA (2000) Influence of oviductal cells and conditioned medium on porcine gametes. Zygote 8:139–144
Dobrinski I, Smith TT, Suarez SS, Ball BA (1997) Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol Reprod 56:861–869
Ellington JE, Evenson DP, Wright RW Jr, Jones AE, Schneider CS, Hiss GA, Brisbois RS (1999) Higher-quality human sperm in a sample selectively attach to oviduct (fallopian tube) epithelial cells in vitro. Fertil Steril 71:924–929
Elliott RM, Duncan AE, Watson PF, Holt WVFA (2001) Enhanced boar sperm viability with porcine oviductal apical plasma membranes (APM). Reprod Abstr Ser 27:33
Elliott RM, Lloyd RE, Fazeli A, Sostaric E, Georgiou AS, Satake N, Watson PF, Holt WV (2009) Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 137:191–203
Fabrega A, Puigmule M, Yeste M, Casas I, Bonet S, Pinart E (2011) Impact of epididymal maturation, ejaculation and in vitro capacitation on tyrosine phosphorylation patterns exhibited of boar (Sus domesticus) spermatozoa. Theriogenology 76:1356–1366
Fazeli A, Duncan AE, Watson PF, Holt WV (1999) Sperm-oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod 60:879–886
Fazeli A, Watson P, Holt W (2000) Isthmic apical plasma membrane preparations maintain boar sperm viability in vitro in a dose dependent manner. Biol Reprod (Suppl 1):388
Fong-Ngern K, Chiangjong W, Thongboonkerd V (2009) Peeling as a novel, simple, and effective method for isolation of apical membrane from intact polarized epithelial cells. Anal Biochem 395:25–32
García Del Moral R (1993) Laboratorio de anatomía patológica. nteramericana-McGraw Hill, Argentina
Gerke V, Weber K (1984) Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J 3:227–233
Grasa P, Colas C, Gallego M, Monteagudo L, Muino-Blanco T, Cebrian-Perez JA (2009) Changes in content and localization of proteins phosphorylated at tyrosine, serine and threonine residues during ram sperm capacitation and acrosome reaction. Reproduction 137:655–667
Gualtieri R, Boni R, Tosti E, Zagami M, Talevi R (2005) Intracellular calcium and protein tyrosine phosphorylation during the release of bovine sperm adhering to the fallopian tube epithelium in vitro. Reproduction 129:51–60
Gualtieri R, Talevi R (2000) In vitro-cultured bovine oviductal cells bind acrosome-intact sperm and retain this ability upon sperm release. Biol Reprod 62:1754–1762
Holt WV, Elliott RM, Fazeli A, Satake N, Watson PF (2005) Validation of an experimental strategy for studying surface-exposed proteins involved in porcine sperm-oviduct contact interactions. Reprod Fertil Dev 17:683–692
Ignotz GG, Cho MY, Suarez SS (2007) Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol Reprod 77:906–913
Killian GJ (2004) Evidence for the role of oviduct secretions in sperm function, fertilization and embryo development. Anim Reprod Sci 82–83:141–153
Kouba AJ, Abeydeera LR, Alvarez IM, Day BN, Buhi WC (2000) Effects of the porcine oviduct-specific glycoprotein on fertilization, polyspermy, and embryonic development in vitro. Biol Reprod 63:242–250
Lefebvre R, Suarez SS (1996) Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol Reprod 54:575–582
Lloyd RE, Elliott RM, Fazeli A, Watson PF, Holt WV (2009) Effects of oviductal proteins, including heat shock 70 kDa protein 8, on survival of ram spermatozoa over 48 h in vitro. Reprod Fertil Dev 21:408–418
Marini PE, Cabada MO (2003) One step purification and biochemical characterization of a spermatozoa-binding protein from porcine oviductal epithelial cells. Mol Reprod Dev 66:383–390
Matsumoto H, Koyama Y, Tanioka A (2003) Interaction of proteins with weak amphoteric charged membrane surfaces: effect of pH. J Colloid Interface Sci 264:82–88
Mburu JN, Einarsson S, Lundeheim N, Rodriguez-Martinez H (1996) Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci 45:109–121
Mburu JN, Rodriguez-Martinez H, Einarsson S (1997) Changes in sperm ultrastructure and localisation in the porcine oviduct around ovulation. Anim Reprod Sci 47:137–148
Parrish JJ, Susko-Parrish J, Winer MA, First NL (1988) Capacitation of bovine sperm by heparin. Biol Reprod 38:1171–1180
Perez FA, Roma SM, Cabada MO, Marini PE (2006) Sperm binding glycoprotein is differentially present surrounding the lumen of isthmus and ampulla of the pig's oviduct. Anat Embryol (Berl) 211:619–624
Petrunkina AM, Friedrich J, Drommer W, Bicker G, Waberski D, Topfer-Petersen E (2001a) Kinetic characterization of the changes in protein tyrosine phosphorylation of membranes, cytosolic Ca2+ concentration and viability in boar sperm populations selected by binding to oviductal epithelial cells. Reproduction 122:469–480
Petrunkina AM, Gehlhaar R, Drommer W, Waberski D, Topfer-Petersen E (2001b) Selective sperm binding to pig oviductal epithelium in vitro. Reproduction 121:889–896
Rodriguez-Martinez H, Saravia F, Wallgren M, Tienthai P, Johannisson A, Vazquez JM, Martinez E, Roca J, Sanz L, Calvete JJ (2005) Boar spermatozoa in the oviduct. Theriogenology 63:514–535
Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE (2007) Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl 65:245–259
Satake N, Elliott RM, Watson PF, Holt WV (2006) Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct. J Exp Biol 209:1560–1572
Smith TT, Nothnick WB (1997) Role of direct contact between spermatozoa and oviductal epithelial cells in maintaining rabbit sperm viability. Biol Reprod 56:83–89
Smith TT, Yanagimachi R (1991) Attachment and release of spermatozoa from the caudal isthmus of the hamster oviduct. J Reprod Fertil 91:567–573
Stuart MA, Fleer GJ, Lyklema J, Norde W, Scheutjens JM (1991) Adsorption of ions, polyelectrolytes and proteins. Adv Colloid Interface Sci 34:477–535
Talevi R, Gualtieri R (2010) Molecules involved in sperm-oviduct adhesion and release. Theriogenology 73:796–801
Teijeiro JM, Cabada MO, Marini PE (2008) Sperm binding glycoprotein (SBG) produces calcium and bicarbonate dependent alteration of acrosome morphology and protein tyrosine phosphorylation on boar sperm. J Cell Biochem 103:1413–1423
Teijeiro J M, Dapino D D, Marini P E (2011) Porcine oviduct sperm binding glycoprotein and its deleterious effect on sperm: a mechanism for negative selection of sperm? Biol Res (in press)
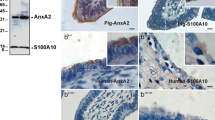
Teijeiro JM, Ignotz GG, Marini PE (2009) Annexin A2 is involved in pig (Sus scrofa) sperm-oviduct interaction. Mol Reprod Dev 76:334–341
Thomas PG, Ball BA, Miller PG, Brinsko SP, Southwood L (1994) A subpopulation of morphologically normal, motile spermatozoa attach to equine oviductal epithelial cell monolayers. Biol Reprod 51:303–309
Thomas PG, Ignotz GG, Ball BA, Brinsko SP, Currie WB (1995) Effect of coculture with stallion spermatozoa on de novo protein synthesis and secretion by equine oviduct epithelial cells. Am J Vet Res 56:1657–1662
Tienthai P, Johannisson A, Rodriguez-Martinez H (2004) Sperm capacitation in the porcine oviduct. Anim Reprod Sci 80:131–146
Töpfer-Petersen E, Ekhlasi-Hundrieser M, Tsolova M (2008) Glycobiology of fertilization in the pig. Int J Dev Biol 52:717–736
Töpfer-Petersen E, Wagner A, Friedrich J, Petrunkina A, Ekhlasi-Hundrieser M, Waberski D, Drommer W (2002) Function of the mammalian oviductal sperm reservoir. J Exp Zool 292:210–215
Yeste M, Lloyd RE, Badia E, Briz M, Bonet S, Holt WV (2009) Direct contact between boar spermatozoa and porcine oviductal epithelial cell (OEC) cultures is needed for optimal sperm survival in vitro. Anim Reprod Sci 113:263–278
Acknowledgements
We thank Dr. Laura Trumper for providing antibodies anti-Na+/K+-ATPase α1 and Dr. Volker Gerke for providing anti-annexin A2 antibodies. We also thank Frigorífico Paladini SA for the oviducts. This work was supported in part by the ANPCyT-BID program PICT 01–15092 of Argentina. Patricia E. Marini is a member of the research career CIC-UNR.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
(AVI 27909 kb)
(AVI 27909 kb)
Rights and permissions
About this article
Cite this article
Teijeiro, J.M., Marini, P.E. Apical membranes prepared by peeling from whole porcine oviducts interact with homologous sperm. Cell Tissue Res 348, 213–223 (2012). https://doi.org/10.1007/s00441-012-1338-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1338-3