Abstract
Uterine epithelial cells (UECs) undergo extensive morphological remodelling in preparation for an implanting blastocyst. This remodelling involves changes in the actin cytoskeleton and surface structures including microvilli. Ezrin and ezrin-radixin-moesin-binding protein-50-kDa (EBP50) link actin filaments to intra-membranous adhesion molecules and are important molecules in polarised epithelia. The current study is the first to describe the colocalisation and molecular association of ezrin and EBP50 in rat UECs by using immunofluorescence microscopy and immunoprecipitation techniques. These proteins have also been localised in relation to uterine epithelial cytoskeletal rearrangement during early pregnancy in the rat and to the effect of apical surface contact between opposing epithelial cells, blastocyst contact and contact with a silicon filament. Immunofluorescence microscopy has revealed that ezrin and EBP50 respond to contact between opposing epithelial cells and increase apically on day 6 of pregnancy. This apical distribution is also observed in UECs in contact with a silicon filament. Ezrin and EBP50 are however absent within the implantation chamber itself, seemingly mimicking the events that take place in leucocyte-endothelium binding. Thus, ezrin and EBP50 occur apically in UECs at the time of implantation in the rat and in response to a substitute blastocyst (filament) suggesting a role for these proteins in the cytoskeletal rearrangements that facilitate uterine receptivity and blastocyst-epithelial adhesion. Their loss within the implantation chamber possibly allows the subsequent invasion of the embryo.




Similar content being viewed by others
References
Aplin JD (1997) Adhesion molecules in implantation. Rev Reprod 2:84–93
Auerbach SD, Yang L, Luscinskas FW (2007) Endothelial ICAM-1 functions in adhesion and signaling during leukocyte recruitment. In: Ley K (ed) Adhesion molecules: function and inhibition. Birkhauser, Basal, pp 99-116
Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (2002) Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 157:1233–1245
Berryman M, Franck Z, Bretscher A (1993) Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci 105:1025–1043
Berryman M, Gary R, Bretscher A (1995) Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J Cell Biol 131:1231–1242
Bretscher A, Reczek D, Berryman M (1997) Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci 110:3011–3018
Bretscher A, Chambers D, Nguyen R, Reczek D (2000) ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 16:113–143
Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C (2005) Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J 19:1056–1060
Enders AC, Schlafke S (1967) A morphological analysis of the early implantation stages in the rat. Am J Anat 120:185–226
Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O (1998) Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem 273:21893–21900
Ingraffea J, Reczek D, Bretscher A (2002) Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur J Cell Biol 81:61–68
Kaneko Y, Lecce L, Murphy CR (2009) Ovarian hormones regulate expression of the focal adhesion proteins, talin and paxillin, in rat uterine luminal but not glandular epithelial cells. Histochem Cell Biol 132:613–622
LaLonde DP, Garbett D, Bretscher A (2010) A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol Biol Cell 21:1519–1529
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lecce L, Kaneko Y, Murphy CR (2010) CD43 is relocated from the basal to the apical plasma membrane of rat uterine epithelial cells by progesterone. Histochem Cell Biol 133:549–555
Luxford KA, Murphy CR (1989) Cytoskeletal alterations in the microvilli of uterine epithelial cells during early pregnancy. Acta Histochem 87:131–136
Luxford KA, Murphy CR (1992a) Changes in the apical microfilaments of rat uterine epithelial cells in response to estradiol and progesterone. Anat Rec 233:521–526
Luxford KA, Murphy CR (1992b) Reorganization of the apical cytoskeleton of uterine epithelial cells during early pregnancy in the rat: a study with myosin subfragment 1. Biol Cell 74:195–202
Matsumoto H, Daikoku T, Wang H, Sato E, Dey SK (2004) Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation. Biol Reprod 70:729–736
Morales FC, Takahashi Y, Kreimann EL, Georgescu MM (2004) Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA 101:17705–17710
Saotome I, Curto M, McClatchey AI (2004) Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell 6:855–864
Serrador JM, Nieto M, Alonso-Lebrero JL, Pozo MA del, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, Gonzalez-Amaro R, Sanchez-Mateos P, Sanchez-Madrid F (1998) CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 91:4632–4644
Shenolikar S, Voltz JW, Cunningham R, Weinman EJ (2004) Regulation of ion transport by the NHERF family of PDZ proteins. Physiology 19:362–369
Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, Cohen WA, Ramesh V, Louis DN (2001) NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol 158:57–62
Terry V, Shaw TJ, Shorey CD, Murphy CR (1996) Actin-binding proteins undergo major alterations during the plasma membrane transformation in uterine epithelial cells. Anat Rec 246:71–77
Tsukita S, Yonemura S (1999) Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem 274:34507–34510
Weinman EJ, Steplock D, Shenolikar S (2003) NHERF-1 uniquely transduces the cAMP signals that inhibit sodium-hydrogen exchange in mouse renal apical membranes. FEBS Lett 536:141–144
Yonemura S, Tsukita S (1999) Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol 145:1497–1509
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
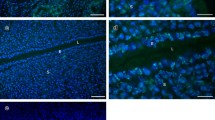
Fig. S1
Immunolocalisation of ezrin in the uterus of a non-pregnant rat. This image displays similar protein staining to that at day 1 of pregnancy. The uterine epithelial cells (UECs) display an abundance of cytoplasmic staining of ezrin, in addition to an apical concentration of ezrin in cells that contact opposing cells (arrowhead). This apical band is absent from UECs (ep) that face the lumen (L). Bar 30 μm. (GIF 68 kb)
Rights and permissions
About this article
Cite this article
Lecce, L., Lindsay, L.A. & Murphy, C.R. Ezrin and EBP50 redistribute apically in rat uterine epithelial cells at the time of implantation and in response to cell contact. Cell Tissue Res 343, 445–453 (2011). https://doi.org/10.1007/s00441-010-1088-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1088-z