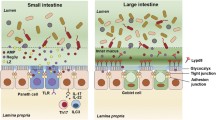
Abstract
The mucosal surfaces of the respiratory, gastrointestinal and urogenital tracts are covered by a layer of epithelial cells that are responsible for sensing and promoting a host immune response in order to establish the limits not only for commensal microorganisms but also for foreign organisms or particles. This is a remarkable task as the human body represents a composite of about 10 trillion human-self cells plus non-self cells from autochthonous or indigenous microbes that outnumber human cells 10:1. Hence, the homeostasis of epithelial cells that line mucosal surfaces relies on a fine-tuned immune system that patrols the boundaries between human and microbial cells. In the case of the intestine, the epithelial layer is composed of at least six epithelial cell lineages that act as a physiological barrier in addition to aiding digestion and the absorption of nutrients, water and electrolytes. In this review, we highlight the immense role of the intestinal epithelium in coordinating the mucosal innate immune response.

Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- CARD:
-
Caspase recruitment domain
- CrD:
-
Crohn’s disease
- GALT:
-
Gut-associated lymphoid tissue
- GI:
-
Gastrointestinal
- IBD:
-
Irritable bowel disease
- IL:
-
Interleukins
- IgA:
-
Immunoglobulin A
- pIgAR:
-
Polimeric immunoglobulin A receptor
- LPS:
-
Lipopolysaccharide
- M cells:
-
Microfold cells
- MAPK:
-
Mitogen-activated protein kinases
- NF-κB:
-
Nuclear factor kappa-B
- NK:
-
Natural killer
- NOD:
-
Nucleotide-binding oligomerization domain
- NLRs:
-
NOD-like receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- PYD:
-
Pyrin domain
- TLR:
-
Toll-like receptors
References
Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M (2001) Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 167:1609–1616
Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S (1996) Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol 133:43–47
Apodaca G, Bomsel M, Arden J, Breitfeld PP, Tang K, Mostov KE (1991) The polymeric immunoglobulin receptor. A model protein to study transcytosis. J Clin Invest 87:1877–1882
Arijs I, De Hertogh G, Lemaire K, Quintens R, Van Lommel L, Van Steen K, Leemans P, Cleynen I, Van Assche G, Vermeire S, Geboes K, Schuit F, Rutgeerts P (2009) Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS ONE 4:e7984
Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK (2005) Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J Cell Biol 170:21–26
Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG (1995) hBD-1: a novel beta-defensin from human plasma. FEBS Lett 368:331–335
Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA (2010) Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6:e1000902
Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, Kobayashi KS (2010) Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci USA 107:14739–14744
Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE (2007) Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol 292:G1770–G1783
Cario E, Podolsky DK (2000) Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68:7010–7017
Cario E, Brown D, McKee M, Lynch-Devaney K, Gerken G, Podolsky DK (2002) Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 160:165–173
Charrier L, Merlin D (2006) The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest 86:538–546
Chen LM, Hobbie S, Galan JE (1996) Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115–2118
Cho JH (2008) The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8:458–466
Corazziari ES (2009) Intestinal mucus barrier in normal and inflamed colon. J Pediatr Gastroenterol Nutr 48(Suppl 2):S54–S55
de Repentigny L, Aumont F, Bernard K, Belhumeur P (2000) Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun 68:3172–3179
De Smet K, Contreras R (2005) Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27:1337–1347
Diamond G, Bevins CL (1998) Beta-defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol 88:221–225
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102:448–455
Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL (2007) Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun 75:2572–2579
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753
Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE (2000) Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355:1518–1519
Fehlbaum P, Rao M, Zasloff M, Anderson GM (2000) An essential amino acid induces epithelial beta-defensin expression. Proc Natl Acad Sci USA 97:12723–12728
Fleming A (1922) On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond [Biol] 39:306–317
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S (1994) Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127:1617–1626
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550
Fyderek K, Strus M, Kowalska-Duplaga K, Gosiewski T, Wedrychowicz A, Jedynak-Wasowicz U, Sladek M, Pieczarkowski S, Adamski P, Kochan P, Heczko PB (2009) Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol 15:5287–5294
Ganz T (1999) Defensins and host defense. Science 286:420–421
Garcia JR, Jaumann F, Schulz S, Krause A, Rodriguez-Jimenez J, Forssmann U, Adermann K, Kluver E, Vogelmeier C, Becker D, Hedrich R, Forssmann WG, Bals R (2001) Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res 306:257–264
Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167:1882–1885
Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ (2001) CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2:736–742
Grutzkau A, Hanski C, Hahn H, Riecken EO (1990) Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011–1015
Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF (2002) Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 70:953–963
Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L, Kagnoff MF (2003) Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 125:1613–1625
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745
Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK (2003) CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993–1000
Horiguchi Y, Senda T, Sugimoto N, Katahira J, Matsuda M (1995) Bordetella bronchiseptica dermonecrotizing toxin stimulates assembly of actin stress fibers and focal adhesions by modifying the small GTP-binding protein rho. J Cell Sci 108:3243–3251
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752
Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol 174:4901–4907
Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G (2001) Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7:180–185
Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 147:1351–1363
Jia HP, Schutte BC, Schudy A, Linzmeier R, Guthmiller JM, Johnson GK, Tack BF, Mitros JP, Rosenthal A, Ganz T, McCray PB Jr (2001) Discovery of new human beta-defensins using a genomics-based approach. Gene 263:211–218
Jones BD, Ghori N, Falkow S (1994) Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med 180:15–23
Kalabis J, Rosenberg I, Podolsky DK (2006) Vangl1 protein acts as a downstream effector of intestinal trefoil factor (ITF)/TFF3 signaling and regulates wound healing of intestinal epithelium. J Biol Chem 281:6434–6441
Kawai T, Akira S (2006) TLR signaling. Cell Death Differ 13:816–825
Keshav S (2006) Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol 80:500–508
Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK (1995) Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 109:516–523
Knutson L, Ahrenstedt O, Odlind B, Hallgren R (1990) The jejunal secretion of histamine is increased in active Crohn’s disease. Gastroenterology 98:849–854
Kufer TA, Kremmer E, Adam AC, Philpott DJ, Sansonetti PJ (2008) The pattern-recognition molecule Nod1 is localized at the plasma membrane at sites of bacterial interaction. Cell Microbiol 10:477–486
Leonova L, Kokryakov VN, Aleshina G, Hong T, Nguyen T, Zhao C, Waring AJ, Lehrer RI (2001) Circular minidefensins and posttranslational generation of molecular diversity. J Leukoc Biol 70:461–464
Lyons S, Wang L, Casanova JE, Sitaraman SV, Merlin D, Gewirtz AT (2004) Salmonella typhimurium transcytoses flagellin via an SPI2-mediated vesicular transport pathway. J Cell Sci 117:5771–5780
Marchetti M, Sirard JC, Sansonetti P, Pringault E, Kerneis S (2004) Interaction of pathogenic bacteria with rabbit appendix M cells: bacterial motility is a key feature in vivo. Microbes Infect 6:521–528
Marra A, Isberg RR (1997) Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun 65:3412–3421
Mastroianni JR, Ouellette AJ (2009) Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem 284:27848–27856
Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, Hediger MA, Madara JL (2001) Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120:1666–1679
Mostov KE (1994) Transepithelial transport of immunoglobulins. Annu Rev Immunol 12:63–84
Neutra MSP, Kraehenbuhl JP (2003) Role of intestinal M cells in microbial pathogenesis. In: Hecht G (ed) Microbial pathogenesis and the intestinal cell. ASM, Washington, DC, pp 23–42
Nuding S, Fellermann K, Wehkamp J, Stange EF (2007) Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut 56:1240–1247
Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL (1995) Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA 92:10629–10633
Nusrat A, Eichel-Streiber C von, Turner JR, Verkade P, Madara JL, Parkos CA (2001) Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69:1329–1336
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606
Oswald E, Sugai M, Labigne A, Wu HC, Fiorentini C, Boquet P, O’Brien AD (1994) Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc Natl Acad Sci USA 91:3814–3818
Ouellette AJ, Selsted ME (1996) Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J 10:1280–1289
Pauleau AL, Murray PJ (2003) Role of nod2 in the response of macrophages to Toll-like receptor agonists. Mol Cell Biol 23:7531–7539
Perdomo JJ, Gounon P, Sansonetti PJ (1994) Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest 93:633–643
Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS (2009) Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA 106:15813–15818
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088
Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D (1999) Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189:615–625
Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG (1995) Mucosal histamine content and histamine secretion in Crohn’s disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol 108:127–133
Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC (2002) Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 4:367–381
Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL (2003) Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522–526
Salzman NH, Underwood MA, Bevins CL (2007) Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 19:70–83
Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11:76–83
Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M (1996) Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun 64:2752–2764
Schröder JM (1999) Epithelial antimicrobial peptides: innate local host response elements. Cell Mol Life Sci 56:32–46
Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu DZ, Deitch EA (2010) Loss of the intestinal mucus layer in the normal rat causes gut injury, but not toxic mesenteric lymph nor lung injury. Shock 34:475–481
Simonovic I, Rosenberg J, Koutsouris A, Hecht G (2000) Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol 2:305–315
Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S (1999) Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147:195–204
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335–376
Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498–502
Tollin M, Bergman P, Svenberg T, Jornvall H, Gudmundsson GH, Agerberth B (2003) Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24:523–530
Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 105:20858–20863
Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB (2004) hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127:1401–1409
Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H Jr, Fellermann K, Ganz T, Stange EF, Bevins CL (2005) Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA 102:18129–18134
Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC (1999) Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113–117
Wittchen ES, Haskins J, Stevenson BR (1999) Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem 274:35179–35185
Zhao C, Wang I, Lehrer RI (1996) Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 396:319–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maldonado-Contreras, A.L., McCormick, B.A. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res 343, 5–12 (2011). https://doi.org/10.1007/s00441-010-1082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1082-5