Abstract
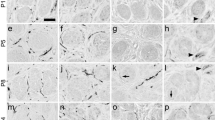
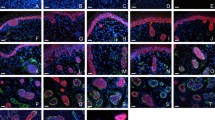
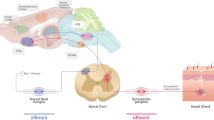
The evolution of aquaporin-5 (AQP5) expression during postnatal development has not been defined in the sweat gland. Previous studies have suggested that AQP isoforms in several peripheral targets are regulated by a neural mechanism. We have examined, in rat sweat glands, the expression of AQP5 during postnatal development and the effects of denervation on AQP5 expression. Both AQP5 mRNA and protein begin to be expressed at postnatal day 10, before sweat-secretory responsiveness first appears; this expression coincides with the occurrence of vasoactive intestinal peptide (VIP) immunoreactivity. Early noradrenergic and later cholinergic interaction between sweat glands and their innervation are disrupted by neonatal chemical sympathectomy or postnatal severance of the sciatic nerve. Examination of such denervated developing rats has shown that secretory responsiveness fails to arise later in the adults, and AQP5 immunostaining increases in the denervated glands, whereas gland morphogenesis and the occurrence of AQP5 expression proceed normally. Immunobloting has revealed an increase of AQP5 abundance after the denervated mature glands lose their secretory ability. These findings suggest that AQP5 protein is necessary for sweat secretion, and that the expression of AQP5 in rat sweat glands is independent of sympathetic innervation. Our data also indicate that factor(s) regulating the normal morphological development of sweat gland might be responsible for controlling AQP5 expression.






Similar content being viewed by others
References
Bondy C, Chin E, Smith BL, Preston GM, Agre P (1993) Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci USA 90:4500–4504
Bovell DL, Lindsay SL, Corbett AD, Steel C (2006) Immunolocalization of aquaporin-5 expression in sweat gland cells from normal and anhidrotic horses. Vet Dermatol 17:17–23
Ekstrom J (1980) Sensitization of the rat parotid gland to secretagogues following either parasympathetic denervation or sympathetic denervation or decentralization. Acta Physiol Scand 108:253–261
Ernsberger U, Rohrer H (1999) Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res 297:339–361
Fu X, Li J, Sun X, Sun T, Sheng Z (2005) Epidermal stem cells are the source of sweat glands in human fetal skin: evidence of synergetic development of stem cells, sweat glands, growth factors, and matrix metalloproteinases. Wound Repair Regen 13:102–108
Grant MP, Landis SC (1991) Developmental expression of muscarinic cholinergic receptors and coupling to phospholipase C in rat sweat glands are independent of innervation. J Neurosci 11:3772–3782
Grant MP, Landis SC, Siegel RE (1991) The molecular and pharmacological properties of muscarinic cholinergic receptors expressed by rat sweat glands are unaltered by denervation. J Neurosci 11:3763–3771
Grant MP, Francis NJ, Landis SC (1995) The role of acetylcholine in regulating secretory responsiveness in rat sweat glands. Mol Cell Neurosci 6:32–42
Guidry G, Landis SC (1995) Sympathetic axons pathfind successfully in the absence of target. J Neurosci 15:7565–7574
Guidry G, Landis SC (1998) Target-dependent development of the vesicular acetylcholine transporter in rodent sweat gland innervation. Dev Biol 199:175–184
Hill AE, Shachar-Hill B, Shachar-Hill Y (2004) What are aquaporins for? J Membr Biol 197:1–32
Hoffert JD, Leitch V, Agre P, King LS (2000) Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem 275:9070–9077
Ishikawa Y, Cho G, Yuan Z, Inoue N, Nakae Y (2006) Aquaporin-5 water channel in lipid rafts of rat parotid glands. Biochim Biophys Acta 1758:1053–1060
Jimi T, Wakayama Y, Murahashi M, Shibuya S, Inoue M, Hara H, Matsuzaki Y, Uemura N (2000) Aquaporin 4: lack of mRNA expression in the rat regenerating muscle fiber under denervation. Neurosci Lett 291:93–96
Jimi T, Wakayama Y, Matsuzaki Y, Hara H, Inoue M, Shibuya S (2004) Reduced expression of aquaporin 4 in human muscles with amyotrophic lateral sclerosis and other neurogenic atrophies. Pathol Res Pract 200:203–209
Jung JY, Byun KO, Kim WJ (2003) Altered expression of aquaporins in rat submandibular glands after parasympathetic denervation. Korean J Physiol Pharmacol 7:97–101
Kennedy WR, Sakuta M (1984) Collateral reinnervation of sweat glands. Ann Neurol 15:73–78
King LS, Nielsen S, Agre P (1997) Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat. Am J Physiol 273:C1541–C1548
King LS, Kozono D, Agre P (2004) From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5:687–698
Landis SC (1999) Development of muscarinic receptors and regulation of secretory responsiveness in rodent sweat glands. Life Sci 64:381–385
Landis SC, Keefe D (1983) Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol 98:349–372
Landis SC, Siegel RE, Schwab M (1988) Evidence for neurotransmitter plasticity in vivo. II. Immunocytochemical studies of rat sweat gland innervation during development. Dev Biol 126:129–140
Lee J, Yoo K, Kim SW, Jung KH, Ma SK, Lee YK, Kim WY, Kim J, Choi KC (2006) Decreased expression of aquaporin water channels in denervated rat kidney. Nephron Physiol 103:170–178
Li J, Fu X, Sun X, Sun T, Sheng Z (2002) The interaction between epidermal growth factor and matrix metalloproteinases induces the development of sweat glands in human fetal skin. J Surg Res 106:258–263
Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S (2002) Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci USA 99:511–516
Saga K (2002) Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem 37:323–386
Schäfer MKH, Schütz B, Weihe E, Eiden L (1997) Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc Natl Acad Sci USA 94:4149–4154
Shikiji T, Minami M, Inoue T, Hirose K, Oura H, Arase S (2003) Keratinocytes can differentiate into eccrine sweat ducts in vitro: involvement of epidermal growth factor and fetal bovine serum. J Dermatol Sci 33:141–150
Sidhaye VK, Guler AD, Schweitzer KS, D’Alessio F, Caterina MJ, King LS (2006) Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc Natl Acad Sci USA 103:4747–4752
Song Y, Sonawane N, Verkman AS (2002) Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J Physiol (Lond) 541:561–568
Stevens LM, Landis SC (1987) Development and properties of the secretory response in rat sweat glands: relationship to the induction of cholinergic function in sweat gland innervation. Dev Biol 123:179–190
Stevens LM, Landis SC (1988) Developmental interactions between sweat glands and the sympathetic neurons which innervate them: effects of delayed innervation on neurotransmitter plasticity and gland maturation. Dev Biol 130:703–720
Takata K, Matsuzaki T, Tajika Y (2004) Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 39:1–83
Talamo BR, Adler SC, Burt DR (1979) Parasympathetic denervation decreases muscarinic receptor binding in rat parotid. Life Sci 24:1573–1580
Tian H, Habecker B, Guidry G, Gurtan A, Rios M, Roffler-Tarlov S, Landis SC (2000) Catecholamines are required for the acquisition of secretory responsiveness by sweat glands. J Neurosci 20:7362–7369
Vilches JJ, Navarro X (2002) New silicones for the evaluation of sudomotor function with the impression mold technique. Clin Auton Res 12:20–23
Vilches JJ, Navarro X, Verdu E (1995) Functional sudomotor responses to cholinergic agonists and antagonists in the mouse. J Auton Nerv Syst 55:105–111
Yodlowski ML, Fredieu JR, Landis SC (1984) Neonatal 6-hydroxydopamine treatment eliminates cholinergic sympathetic innervation and induces sensory sprouting in rat sweat glands. J Neurosci 4:1535–1548
Acknowledgments
We thank Prof. Zhi-Ren Rao, Hong-Ge Jia, and Liang-Wei Chen for morphological expertise and helpful advice, and Li Duan and Rong Cao for skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Huang, YG., He, H. et al. Postnatal expression and denervation induced up-regulation of aquaporin-5 protein in rat sweat gland. Cell Tissue Res 329, 25–33 (2007). https://doi.org/10.1007/s00441-007-0399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0399-1