Abstract
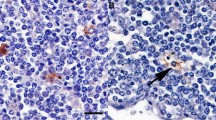
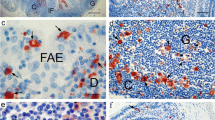
During preclinical stages of cattle orally infected with bovine spongiform encephalopathy (BSE), the responsible agent is confined to ileal Peyer’s patches (IPP), namely in nerve fibers and in lymph follicles, before reaching the peripheral and central nervous systems. No infectivity has been reported in other bovine lymphoid organs, including jejunal Peyer’s patches (JPP). To determine the potential sites for prion neuroinvasion in IPP, we analyzed the mucosal innervation and the interface between nerve fibers and follicular dendritic cells (FDC), two dramatic influences on neuroinvasion. Bovine IPP were studied at three ages, viz., newborn calves, calves less than 12 months old, and bovines older than 24 months, and the parameters obtained were compared with those of JPP. No differences in innervation patterns between IPP and JPP were found. The major difference observed was that, in calves of less than 12 months, IPP were the major mucosal-associated lymphoid organ that possessed a large number of follicles with extended FDC networks. Using a panel of antibodies, we showed that PP in 24-month-old bovines were highly innervated at various strategic sites assumed to be involved in the invasion and replication of the BSE pathogen: the suprafollicular dome, T cell area, and germinal centers. In PP in calves of less than 12 months old, no nerve fibers positive for the neurofilament markers NF-L (70 kDa) and NF-H (200 kDa) were observed in contact with FDC. Thus, in view of the proportion of these protein subunits present in neurofilaments, the innervation of the germinal centers can be said to be an age-dependent dynamic process. This variation in innervation might influence the path of neuroinvasion and, thus, the susceptibility of bovines to the BSE agent.





Similar content being viewed by others
References
Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, Keulen L van, Schelcher F, Elsen JM, Lantier F (2000) Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol 81:3115–3126
Balemba OB, Mbassa GK, Semuguruka WD, Assey RJ, Kahwa CK, Hay-Schmidt A, Dantzer V (1999) The topography, architecture and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat 195:1–9
Blattler T, Brandner S, Raeber AJ, Klein MA, Voigtlander T, Weissmann C, Aguzzi A (1997) PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389:69–73
Bradley R (1999) BSE transmission studies with particular reference to blood. Dev Biol Stand 99:35–40
Brandner S, Raeber A, Sailer A, Blattler T, Fischer M, Weissmann C, Aguzzi A (1996) Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proc Natl Acad Sci USA 93:13148–13151
Brown KL, Stewart K, Ritchie DL, Mabbott NA, Williams A, Fraser H, Morrison WI, Bruce ME (1999) Scrapie replication in lymphoid tissues depends on prion protein-expression follicular dendritic cells. Nat Med 5:1308–1312
Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ (1997) Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501
Bruce ME, McConnell I, Will RG, Ironside JW (2001) Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 358:498–501
Collinge J (1999) Variant Creutzfeldt-Jakob disease. Lancet 354:317–323
Defaweux V, Dorban G, Demonceau C, Piret J, Jolois O, Thellin O, Thielen C, Heinen E, Antoine N (2005) Interfaces between dendritic cells, other immune cells, and nerve fibres in mouse Peyer’s patches: potential sites for neuroinvasion in prion diseases. Microsc Res Tech 66:1–9
Farquhar CF, Dornan J, Moore RC, Somerville RA, Tunstall AM, Hope J (1996) Protease-resistant PrP deposition in brain and non-central nervous system tissues of a murine model of bovine spongiform encephalopathy. J Gen Virol 77:1941–1946
Feher E, Fodor M, Burnstock G (1992) Distribution of somatostatin-immunoreactive nerve fibres in Peyer’s patches. Gut 33:1195–1198
Follet J, Lemaire-Vieille C, Blanquet-Grossard F, Podevin-Dimster V, Lehmann S, Chauvin JP, Decavel JP, Varea R, Grassi J, Fontes M, Cesbron JY (2002) PrP expression and replication by Schwann cells: implications in prion spreading. J Virol 76:2434–2439
Glatzel M, Aguzzi A (2000) PrP(C) expression in the peripheral nervous system is a determinant of prion neuroinvasion. J Gen Virol 81:2813–2821
Glatzel M, Heppner FL, Albers KM, Aguzzi A (2001) Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 39:25–34
Groschup MH, Beekes M, McBride PA, Hardt M, Hainfellner JA, Budka H (1999) Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol 98:453–457
Hadlow WJ, Kennedy RC, Race RE (1982) Natural infection of Suffolk sheep with scrapie virus. J Infect Dis 146:657–664
Haik S, Faucheux BA, Sazdovitch V, Privat N, Kemeny JL, Perret-Liaudet A, Hauw JJ (2003) The sympathetic nervous system is involved in variant Creutzfeldt-Jakob disease. Nat Med 9:1121–1123
Heggebo R, Press CM, Gunnes G, Gonzales L, Jeffrey M (2000) Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. J Gen Virol 83:479–489
Heggebo R, Gonzalez L, Press CM, Gunnes G, Espenes A, Jeffrey M (2003) Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. J Gen Virol 84:1327–1338
Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl JP, Aguzzi A (2001) Transepithelial prion transport by M cells. Nature 7:976–977
Ingrosso L, Pisani F, Pocchiari M (1999) Transmission of the 263K scrapie strain by the dental route. J Gen Virol 80:3043–3047
Jeffrey M, McGovern G, Goodsir CM, Brown KL, Bruce ME (2000) Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol 191:323–332
Joiner S, Linehan JM, Brandner S, Wadsworth JD, Collinge J (2005) High levels of disease related prion protein in the ileum in variant Creutzfeldt-Jakob disease. Gut 54:1506–1508
Krammer HJ, Kuhnel W (1993) Topography of the enteric nervous system in Peyer’s patches of the porcine small intestine. Cell Tissue Res 272:267–272
Kulkarni-Narla A, Beitz AJ, Brown DR (1999) Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res 298:275–286
Legname G, Baskakov IV, NGuyen HO, Riesner HO, Cohen FE, DeArmond SJ, Prusiner SB (2004) Synthetic mammalian prions. Science 305:673–676
Mabbott AN, Mackay F, Minns F, Bruce ME (2000) Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med 6:719–720
Madden KS, Bellinger DL, Felten SY, Snyder E, Maisa ME, Felten DL (1997) Alterations in sympathetic innervation of thymus and spleen in aged mice. Mech Ageing Dev 94:165–175
Madden KS, Thyagarajan S, Felten DL (1998) Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann N Y Acad Sci 840:262–268
Maignien T, Shakweh M, Calvo P, Marce D, Salès N, Fattal E, Deslys JP, Couvreur P, Lasmezas CI (2005) Role of gut macrophages in mice orally contaminated with scrapie or BSE. Int J Pharm 298:293–304
McBride PA, Eikelenboom P, Kraal G, Fraser H, Bruce ME (1992) PrP protein is associated with follicular dendritic cells of spleen and lymph nodes in uninfected and scrapie-infected mice. J Pathol 168:413–418
McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M (2001) Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol 75:9320–9327
Mélot F, Thielen C, Labiet T, Eisher S, Jolois O, Heinen E, Antoine N (2002) Do bovine lymphocytes express a peculiar prion protein? Dev Immunol 9:245–252
Mélot F, Defaweux V, Jolois O, Collard A, Robert B, Heinen E, Antoine N (2004) FDC-B1: a new monoclonal antibody directed against bovine follicular dendritic cells. Vet Immunol Immunopathol 97:1–9
Montrasio F, Frigg R, Glatzel M, Klein MA, Mackay F, Aguzzi A, Weissmann C (2000) Impaired prion replication of mice lacking functional follicular dendritic cells. Science 288:1257–1259
Mutwiri G, Watts T, Lew L, Beskorwayne T, Papp Z, Baca-Estrada ME, Griebel P (1999) Ileal and jejunal Peyer’s patches play distinct roles in mucosal immunity of sheep. Immunology 97:455–461
Pfoch M, Unsicker K (1972) Electron microscopic study on the innervation of Peyer’s patches of the Syrian hamster. Z Zellforsch Mikrosk Anat 123:425–429
Prinz M, Heikenwalder M, Junt T, Glatzel M, Heppner FL, Fu YX, Lipp M, Aguzzi A (2003a) Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425:957–962
Prinz M, Huber G, Macpherson AJ, Heppner FL, Glatzel M, Eugster HP, Wagner N, Aguzzi A (2003b) Oral prion infection requires normal numbers of Peyer’s patches but not of enteric lymphocytes. Am J Pathol 162:1103–1110
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Race R, Oldstone M, Chesebro B (2000) Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J Virol 74:828–833
Reynaud CA, Garcia C, Hein WR, Weill JC (1995) Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell 80:115–125
Somerville RA, Birkett CR, Farquhar CF, Hunter N, Goldmann W, Dornan J, Grover D, Hennion RM, Percy C, Foster J, Jeffrey M (1997) Immunodetection of PrPSc in spleens of some scrapie infected sheep but not BSE-infected cows. J Gen Virol 78:2389–2396
Terry LA, Marsh S, Ryder SJ, Hawkins SA, Wells GA, Spencer YI (2003) Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet Rec 152:387–392
Thielen C, Melot F, Jolois O, Leclercq F, Tsunoda R, Frobert Y, Heinen E, Antoine N (2001) Isolation of bovine follicular dendritic cells allows the demonstration of a particular cellular prion protein. Cell Tissue Res 306:49–55
Thuring CM, Keulen LJ van, Langeveld JP, Vromans ME, Zijderveld FG van, Sweeney T (2005) Immunohistochemical distinction between preclinical bovine spongiform encephalopathy and scrapie infection in sheep. J Comp Pathol 132:59–69
Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J (2001) Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180
Wells GA, Dawson M, Hawkins SA, Green RB, Dexter I, Francis ME, Simmons MM, Austin AR, Horigan MW (1994) Infectivity in the ileum of cattle challenged orally with bovine spongiform encephalopathy. Vet Rec 135:40–41
Wells GA, Hawkins SA, Green RB, Austin AR, Dexter I, Spencer YI, Chaplin MJ, Stack MJ, Dawson M (1998) Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec 142:103–106
Yasuda M, Fujino M, Nasu T, Murakami T (2004) Histological studies on the ontogeny of bovine gut-associated lymphoid tissue: appearance of T cells and development of IgG+ and IgA+ cells in lymphoid follicles. Dev Comp Immunol 28:357–369
Acknowledgements
The SAF 32 antibody was kindly provided by Jacques Grassi. We thank Dr. P. Lausberg for allowing samples to be collected at the local abattoir.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Région Wallonne and by the Leon Frederiq Foundation.
Rights and permissions
About this article
Cite this article
Defaweux, V., Dorban, G., Antoine, N. et al. Neuroimmune connections in jejunal and ileal Peyer’s patches at various bovine ages: potential sites for prion neuroinvasion. Cell Tissue Res 329, 35–44 (2007). https://doi.org/10.1007/s00441-007-0396-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0396-4