Abstract
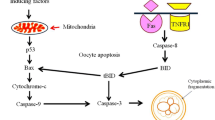
Pyriforms are ovarian follicle nurse cells that undergo apoptosis at the end of previtellogenesis and are completely eliminated by the epithelium. This event is accompanied by the active transfer of organelles and macromolecules to the oocyte via an intercellular bridge. Since it would be a nonsense for damaged mitochondria to reach the oocyte, we have postulated that pyriform cells have adapted their apoptotic machinery to prevent mitochondrial degradation. To verify this hypothesis, we have studied mitochondrial morphology and functionality during follicle cell regression. Cytological and biochemical evidence indicates that mitochondria in pyriforms maintain their size, organization and membrane potential. This clearly indicates that they are not involved in apoptosis signalling/progression. This block would favour both the oocyte, by increasing the pool of organelles available from follicle cells, and also the regressing pyriforms, by maintaining the energy resources required for completion of their nurse function. The block is probably attributable to an over-expression of Bcl-2 and might be carried out by sequestering cytochrome c inside the organelles. As demonstrated by in vitro experiments, the mitochondrial apoptosis pathway can be activated by stress induction, such as serum deprivation, but not following physiological pro-apoptotic signalling, such as treatment with gonadotrophin-releasing hormone.





Similar content being viewed by others
References
Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of Smac/Diablo from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20:6627–6636
Alexandrova O, Schade M, Bottger A, David CN (2005) Oogenesis in Hydra: nurse cells transfer cytoplasm directly to the growing oocyte. Dev Biol 281:91–101
Amsterdam A, Keren-Tal I, Aharoni D, Dantes A, Land-Bracha A, Rimon E, Sasson R, Hirsh L (2003) Steroidogenesis and apoptosis in the mammalian ovary. Steroids 68:861–867
Andreuccetti P, Taddei C, Filosa S (1978) Intercellular bridges between follicle cells and oocyte during the differentiation of follicular epithelium in L. sicula. J Cell Sci 33:341–350
Andreu-Vieyra CV, Habibi HR (2000) Factors controlling ovarian apoptosis. Can J Physiol Pharmacol 78:1003–1012
Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F (1999) Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem 264:687–701
Billig H, Furuta I, Hsueh AJW (1994) Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulosa cells. Endocrinology 134:245–252
Birnbaumer L, Shabibi N, Rivier J, Vale W (1985) Evidence for a physiological role of gonadotropin-releasing hormone (GnRH) or GNRH-like material in the ovary. Endocrinology 116:1367–1370
De Caro M, Indolfi P, Iodice C, Spagnuolo S, Tammaro S, Motta CM (1998) How the ovarian follicle of P. sicula recycles the DNA of its nurse, regressing follicle cells. Mol Reprod Dev 51:421–429
Eguchi Y, Srinivasan A, Tomaselli KJ, Shimizu S, Tsujimoto Y (1999) ATP-dependent steps in apoptotic signal transduction. Cancer Res 59:2174–2181
Filosa S (1973) Biological and cytological aspects of the ovarian cycle in Lacerta sicula Raf. Mon Zool It 7:151–165
Filosa S, Taddei C, Andreuccetti P (1979) The differentiation and the proliferation of the follicle cells during oocyte growth in P. sicula Raf. J Embryol Exp Morphol 54:5–15
Gazourian L, Deragon KL, Chase CF, Pati D, Habibi HR, Sower SA (1997) Characteristics of GnRH binding in the gonads and the effect of lamprey GnRH-I and III on reproduction in the adult lamprey. Gen Comp Endocrinol 108:327–339
Giuliano M, Bellavia G, Lauricella M, D’anneo A, Vassallo B, Vento R, Tesoriere G (2004) Staurosporine-induced apoptosis in Chang liver cells is associated with down-regulation of Bcl-2 and Bcl-XL. Int J Mol Med 13:565–571
Hampton MB, Orrenius S (1997) Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett 414:552–556
Heiskanen KM, Bhat MB, Wang HW, Ma J, Nieminen AL (1999) Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem 274:5654–5658
Kah O, Lethimonier C, Lareyre JJ (2004) Gonadotrophin-releasing hormone (GnRH) in the animal kingdom. J Soc Biol 198:53–60
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixation of high osmolarity for use in electron microscope. J Cell Biol 27:137–148
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Marian J, Conn PM (1983) Subcellular localization of the receptor for gonadotropin-releasing hormone in pituitary and ovarian tissue. Endocrinology 112:104–112
Mazurkiewicz M, Kubrakiewicz J (2001) Intercellular cytoplasm transport during oogenesis of the moth midge, Tinearia alternata Say (Diptera: Psychodidae). Folia Biol 49:205–213
Motta CM, Castriota Scanderberg M, Filosa S, Andreuccetti P (1995) Role of pyriform cells during the growth of oocytes in the lizard P. sicula. J Exp Zool 273:247–256
Motta CM, Filosa S, Andreuccetti P (1996) Regression of the epithelium in late previtellogenic follicle of P. sicula: a case of apoptosis. J Exp Zool 276:111–120
Motta CM, Tammaro S, Cicale A, Indolfi P, Iodice C, Spagnuolo S, Filosa S (2001) Storage in the yolk platelets of low MW DNA produced in the regressing follicle cells. Mol Reprod Dev 59:422–430
Nezis IP, Stravopodis DJ, Papassideri I, Robert-Nicoud M, Margaritis LH (2000) Stage-specific apoptotic patterns during Drosophila oogenesis. Eur J Cell Biol 79:610–620
Oikawa M, Dargan C, Ny T, Hsueh AJW (1990) Expression of gonadotropin-releasing hormone and prothymosin-alpha messenger ribonucleic acid in the ovary. Endocrinology 127:2350–2356
Proskuryakov SY, Konoplyannikov AG, Gabai VL (2003) Necrosis: a specific form of programmed cell death? Exp Cell Res 283:1–16
Qin Y, Vanden Hoek TL, Wojcik K, Anderson T, Li CQ, Shao ZH, Becker LB, Hamann KJ (2004) Caspase-dependent cytochrome c release and cell death in chick cardiomyocytes after simulated ischemia-reperfusion. Am J Physiol Heart Circ Physiol 286:H2280–H2286
Ramakrishnappa N, Rajamahendran R, Lin YM, Leung PC (2005) GnRH in non-hypothalamic reproductive tissues. Anim Reprod Sci 88:95–113
Schultze-Osthoff K, Krammer PH, Droge W (1994) Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J 13:4587–4596
Simm A, Bertsch G, Frank H, Zimmermann U, Hoppe J (1997) Cell death of AKR-2B fibroblasts after serum removal: a process between apoptosis and necrosis. J Cell Sci 110:819–828
Sokal RR, Rohlf FJ (1981) Biometry. Freeman, New York
Springs SL, Diavolitsis VM, Goodhouse J, McLendon GL (2002) The kinetics of translocation of Smac/Diablo from the mitochondria to the cytosol in HeLa cells. J Biol Chem 277:45715–45718
Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM (2002) Bcl-2 and Bcl-XL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/Diablo and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem 277:11345–11351
Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G (1996) Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med 184:1331–1341
Taddei C (1972) Significance of pyriform cells in ovarian follicle of L. sicula. Exp Cell Res 141:562–566
Takizawa T, Robinson JM (1994) Use of 1.4-nm immunogold particles for immunocytochemistry on ultra-thin cryosections. J Histochem Cytochem 42:1615–1623
Tashker JS, Olson M, Kornbluth S (2002) Post-cytochrome c protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol Biol Cell 13:393–401
Tatsumi T, Shiraishi J, Keira N, Akashi K, Mano A, Yamanaka S, Matoba S, Fushiki S, Fliss H, Nakagawa M (2003) Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res 59:428–440
Tilly JL, Billig H, Kowalski KI, Hsuesh AWJ (1992) Epidermal growth factor and basic fibroblast growth factor suppresses the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol 6:1942–1950
Tilly JL, Tilly KI, Kenton ML, Johnson AL (1995) Expression of members of the Bcl-2 gene family in the immature rat ovary: equine chorionic gonadotropin-mediated inhibition of granulosa cell apoptosis is associated with decreased Bax and constitutive Bcl-2 and Bcl-Xlong messenger ribonucleic acid levels. Endocrinology 136:232–241
Troyan MB, Gilman VR, Gay CV (1997) Mitochondrial membrane potential changes in osteoblasts treated with parathyroid hormone and oestradiol. Exp Cell Res 233:274–280
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3:697–707
Uemura T, Namiki T, Kimura A, Yanagisawa T, Minaguchi H (1994) Direct effects of gonadotropin-releasing hormone on the ovary in rats and humans. Horm Res 41 (Suppl 1):7–13
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Yang E, Korsmeyer SJ (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88:386–401
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1125–1136
Yang MY, Rajamahendran R (2000) Morphological and biochemical identification of apoptosis in small, medium, and large bovine follicles and the effects of follicle-stimulating hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured bovine granulosa cells. Biol Reprod 62:1209–1217
Acknowledgements
TEM analyses were carried out at the Centro Interdipartimentale di Ricerche sulle Ultrastrutture Biologiche at Naples and the authors thank staff for their technical assistance. C.M.M. is grateful to Dr. Ted Howell for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
These studies were supported by a grant from the MIUR (PRIN project: Molecular responses of embryonic, differentiated and tumoral cells exposed to cadmium intoxication).
Rights and permissions
About this article
Cite this article
Tammaro, S., Simoniello, P., Filosa, S. et al. Block of mitochondrial apoptotic pathways in lizard ovarian follicle cells as an adaptation to their nurse function. Cell Tissue Res 327, 625–635 (2007). https://doi.org/10.1007/s00441-006-0256-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0256-7