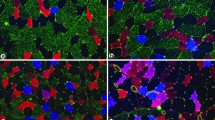
Abstract
The goal of this study was to determine the acute effects of permanent denervation on the length density of the capillary network in rat slow soleus (SOL) and fast extensor digitorum longus (EDL) muscles and the effect of short-lasting reinnervation in slow muscle only. Denervation was performed by cutting the sciatic nerve. Both muscles were excised 2 weeks later. Reinnervation was studied 4 weeks after nerve crush in SOL muscle only. Capillaries and muscle fibres were visualised by triple immunofluorescent staining with antibodies against CD31 and laminin and with fluorescein-labelled Griffonia (Bandeira) simplicifolia lectin. A recently developed stereological approach allowing the estimation of the length of capillaries adjacent to each individual fibre (Lcap/Lfib) was employed. Three-dimensional virtual test grids were applied to stacks of optical images captured with a confocal microscope and their intersections with capillaries and muscle fibres were counted. Interrelationships among capillaries and muscle fibres were demonstrated with maximum intensity projection of the acquired stacks of optical images. The course of capillaries in EDL seemed to be parallel to the fibre axes, whereas in SOL, their preferential direction deviated from the fibre axes and formed more cross-connections among neighbouring capillaries. Lcap/Lfib was clearly reduced in denervated SOL but remained unchanged in EDL, although the muscle fibres significantly atrophied in both muscle types. When soleus muscle was reinnervated, capillary length per unit fibre length was completely restored. The physiological background for the different responses of the capillary network in slow and fast muscle is discussed.


Similar content being viewed by others
References
Artacho-Perula E, Roldán-Villalobos R, Cruz-Orive LM (1999) Application of the fractionator and vertical slices to estimate total capillary length in skeletal muscle. J Anat 195:429–437
Bobinac D, Malnar-Dragojevič D, Bajek S, Šoic-Vranič T, Jerkovič R (2000) Muscle fibre type composition and morphometric properties of denervated rat extensor digitorum longus muscle. Croat Med J 41:294–297
Borisov AB, Huang SK, Carlson BM (2000) Remodelling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec 258:292–304
Carpenter S, Karpati G (1982) Necrosis of capillaries in denervation atrophy of human skeletal muscle. Muscle Nerve 5:250–254
Carry MR, Steven PR, Starcevich JM (1986) Distribution of capillaries in normal and diseased human skeletal muscle. Muscle Nerve 9:445–454
Čebašek V, Kubínová L, Ribarič S, Eržen I (2004) A novel staining method for quantification and 3D visualisation of capillaries and muscle fibres. Eur J Histochem 48:151–158 (http://www.wise-t.com/ias)
Čebašek V, Kubínová L, Ribarič S, Eržen I (2005) Capillary network in slow and fast muscles and in oxidative and glycolytic muscle fibres. Image Anal Stereol 24:51–58 ()
Chernukh AM, Alekseeva NN (1975) Changes in the capillary bed of the skeletal muscles in various periods following nerve section. Biull Eksp Biol Med 80:12–16
Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2002) Resistance vessel remodeling and reparative angiogenesis in the microcirculatory bed of long-term denervated skeletal muscles. Microvasc Res 63:96–114
Delp MD, Armstrong RB (1988) Blood flow in normal and denervated muscle during exercise in conscious rats. Am J Physiol 255:H1509–H1515
Desaki J, Oki S, Matsuda Y, Sakanaka M (2000) Morphological changes of capillaries in the rat soleus muscle following experimental tenotomy. J Electron Microsc (Tokyo) 49:185–193
Desplanches D, Mayet MH, Sempore B, Flandrois R (1987) Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol 63:558–563
Deveci D, Marshall JM, Egginton S (2001) Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol 281:H241–H252
Egginton S, Ross HF (1989) Quantifying capillary distribution in four dimensions. Adv Exp Med Biol 248:271–280
Engel AG, Stonnington HH (1974) Trophic functions of the neuron. II. Denervation and regulation of muscle. Morphological effects of denervation of muscle. A quantitative ultrastructural study. Ann N Y Acad Sci 228:68–88
Goldspink DF (1977) Protein turnover of rat skeletal muscle after denervation. In: Turk V, Marks N (eds) Intracellular protein catabolism II. Proceedings of the 2nd International Symposium on Intracellular Protein Catabolism, 1975, Ljubljana, Yugoslavia. Plenum, New York
Gray SD, Renkin EM (1978) Microvascular supply in relation to fiber metabolic type in mixed skeletal muscles of rabbits. Microvasc Res 16:406–425
Hoofd L, Turek Z, Kubat T, Ringnalda BEM, Kazda S (1985) Variability of intercapillary distance estimated on histological sections of rat heart. Adv Exp Med Biol 191:239–247
Hudlicka O (1991) What makes blood vessels grow? J Physiol (Lond) 444:1–24
Hudlicka O, Tyler KR (1984) The effect of long-term high-frequency stimulation on capillary density and fibre types in rabbit fast muscles. J Physiol (Lond) 353:435–445
Hudlicka O, Brown M, Egginton S (1992) Angiogenesis in skeletal and cardiac muscle. Physiol Rev 72:317–369
Jakubiec-Puka A, Kordowska J, Catani C, Carraro U (1990) Myosin heavy chain isoform composition in striated muscle after denervation and self-reinnervation. Eur J Biochem 193:623–638
Jakubiec-Puka A, Ciechomska I, Morga J, Matusiak A (1999) Contents of myosin heavy chains in denervated slow and fast rat leg muscles. Comp Biochem Physiol [B] 122:355–362
Jozsa L, Jarvinen M, Kvist M, Lehto M, Mikola A (1980) Capillary density of tenotomized skeletal muscles. I. Experimental study in the rat. Eur J Appl Physiol Occup Physiol 44:175–181
Kubínová L, Janáček J (1998) Estimating surface area by the isotropic FAKIR method from thick slices cut in arbitrary direction. J Microsc 191:201–211
Kubínová L, Janáček J, Ribarič S, Čebašek V, Eržen I (2001) Three-dimensional study of the capillary supply of skeletal muscle fibres using confocal microscopy. J Muscle Res Cell Motil 22:217–227
Large J, Tyler KR (1985) Changes in capillary distribution in rat fast muscles following nerve crush and reinnervation. J Physiol (Lond) 362:13–21
Larsen JO, Gundersen HJG, Nielsen J (1998) Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J Microsc 191:238–248
Lexell J (1997) Muscle capillarization: morphological and morphometrical analyses of biopsy samples. Muscle Nerve Suppl 5:S110–S112
Loats JT, Sillau AH, Banchero N (1977) How to quantify skeletal muscle capillarity. Adv Exp Med Biol 94:41–48
Lundby C, Pilegaard H, Andersen JL, Hall G van, Sander M, Calbet JAL (2004) Acclimatization to 4100 m does not change capillary density or mRNA expression of potential angiogenesis regulatory factors in human skeletal muscle. J Exp Biol 207:3865–3871
Mathieu O, Cruz-Orive LM, Hoppeler H, Weibel ER (1983) Estimating length density and quantifying anisotropy in skeletal muscle capillaries. J Microsc 131:131–146
Mathieu-Costello O, Poole DC, Logemann RB (1989) Muscle fiber size and chronic exposure to hypoxia. Adv Exp Med Biol 248:305–311
Mathieu-Costello O, Ellis CG, Potter RF, MacDonald IC, Groom AC (1991) Muscle capillary-to-fiber perimeter ratio: morphometry. Am J Physiol 261:H1617–H1625
Mathieu-Costello O, Agey PJ, Wu L, Hang J, Adair TH (1996) Capillary-to-fiber surface ratio in rat fast-twitch hindlimb muscles after chronic electrical stimulation. J Appl Physiol 80:904–909
Michel RN, Parry DJ, Dunn SE (1996) Regulation of myosin heavy chain expression in adult rat hindlimb muscles during short-term paralysis: comparison of denervation and tetrodotoxin-induced neural inactivation. FEBS Lett 391:39–44
Mrazkova O, Puzanova L (1971) The capillary bed in denervated muscle. Folia Morphol (Praha) 19:71–81
Musacchia XJ, Steffen JM, Fell RD, Dombrowski MJ (1990) Skeletal muscle response to spaceflight, whole body suspension, and recovery in rats. J Appl Physiol 69:2248–2253
Plyley MJ, Groom AC (1975) Geometrical distribution of capillaries in mammalian striated muscle. Am J Physiol 228:1376–1383
Poole DC, Mathieu-Costello O (1990) Effects of hypoxia on capillary orientation in anterior tibialis muscle of highly active mice. Respir Physiol 82:1–10
Poole DC, Mathieu-Costello O, West JB (1989) Capillary tortuosity in rat soleus muscle is not affected by endurance training. Am J Physiol 256:H1110–H1116
Ribarič S, Stefanovska A, Brzin M, Kogovšek M, Krošelj P (1991) Biochemical, morphological, and functional changes during peripheral nerve regeneration. Mol Chem Neuropathol 15:143–157
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205
Sillau AH, Banchero N (1977) Effects of hypoxia on capillary density and fiber composition in rat skeletal muscle. Pflügers Arch 370:227–232
Škorjanc D, Jaschinski F, Heine G, Pette D (1998) Sequential increases in capillarization and mitochondrial enzymes in low-frequency-stimulated rabbit muscle. Am J Physiol 274:C810–C818
Tomori Z (2002) Ellipse version 2.01. ViDiTo, Košice, Slovakia
Tyml K, Mathieu-Costello O, Cheng L, Noble EG (1999) Differential microvascular response to disuse in rat hindlimb skeletal muscles. J Appl Physiol 87:1496–1505
Wroblewski R, Edstrom L, Jakobsson F (1989) Effect of short time denervation on intracellular elemental content and fibre atrophy pattern of slow and fast twitch rat muscle. J Submicrosc Cytol Pathol 21:685–690
Acknowledgements
The authors are grateful to Dr. Katarína Mitrová for her technical assistance with capturing confocal images.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Slovenian Research Agency and the Ministry of Education, Youth and Sport of the Czech Republic (KONTAKT grant no. 19/2005).
Rights and permissions
About this article
Cite this article
Čebašek, V., Radochová, B., Ribarič, S. et al. Nerve injury affects the capillary supply in rat slow and fast muscles differently. Cell Tissue Res 323, 305–312 (2006). https://doi.org/10.1007/s00441-005-0071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0071-6