Abstract
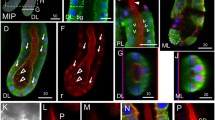
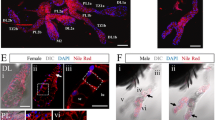
The gene Ag-Aper14 encodes a novel peritrophic matrix (or peritrophic membrane; PM) protein in the mosquito Anopheles gambiae. The Ag-Aper14 protein is merely 89 amino acids long and has a single putative chitin-binding domain. Prior to blood feeding, the Ag-Aper14 protein is stored in secretory vesicles next to the epithelial cell lumenal surface. Immunoelectron microscopy has revealed that Ag-Aper14 co-localizes to the same secretory vesicles as another PM protein, Ag-Aper1, indicating a common mode of regulated secretion. Conversely, Ag-Muc1, an epithelial cell-surface protein, does not co-localize to these secretory vesicles and is detected only on the cell surface. After blood feeding, Ag-Aper14 is secreted and incorporated into the PM that surrounds the ingested blood.










Similar content being viewed by others
References
Berner R, Rudin W, Hecker H (1983) Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (liston) (Insecta: Diptera) under normal and experimental conditions. J Ultrastruct Res 83:195–204
Billingsley PF, Rudin W (1992) The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J Parasitol 78:430–440
Devenport M, Fujioka H, Jacobs-Lorena M (2004) Storage and secretion of the peritrophic matrix protein Ag-Aper1 and trypsin in the midgut of Anopheles gambiae. Insect Mol Biol 13:349–358
Dimopoulos G, Seeley D, Wolf A, Kafatos FC (1998) Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J 17:6115–6123
Flemstrom AA, Garner A, Kivailaaskso E (1993) Gastroduodenal mucosal protection. Physiol Rev 73:823–857
Freyvogel TA, Stäubli W (1965) The formation of the peritrophic membrane in culicidae. Acta Trop 22:118–147
Galun R, Avi-dor Y, Bar-Zeev M (1963) Feeding response in Aedes aegypti: stimulation by adenosine triphosphate. Science 142:1674–1675
Gruber A, Zingales B (1995) Alternative method to remove antibacterial antibodies from antisera used for screening of expression libraries. Biotechniques 19:28–30
Hecker H (1977) Structure and function of midgut epithelial cells in culicidae mosquitoes (Insecta, diptera). Cell Tissue Res 184:321–341
Ibrahim GH, Smartt CT, Kiley LM, Christensen BM (2000) Cloning and characterization of a chitin synthase cDNA from the mosquito Aedes aegypti. Insect Biochem Mol Biol 30:1213–1222
Jacobs-Lorena M, Oo MM (1996) The peritrophic matrix of insects. In: Beaty BJ, Marquardt WC (eds) The biology of disease vectors. University Press of Colorado, Colorado, pp 318–332
Kato N, Dasgupta R, Smartt C, Christensen BM (2002) Glucosamine:fructose-6-phosphate aminotransferase: gene characterization, chitin biosynthesis and peritrophic matrix formation in Aedes aegypti. Insect Mol Biol 11:207–216
Lehane MJ (1997) Peritrophic matrix structure and function. Annu Rev Entomol 42:525–550
Lemos FJA, Cornel AJ, Jacobs-Lorena M (1996) Trypsin and aminopeptidase gene expression is affected by age and food composition in Anopheles gambiae. Insect Biochem Mol Biol 26:651–658
Moskalyk LA, Oo M-M, Jacobs-Lorena M (1996) Peritrophic matrix proteins of Anopheles gambiae and Aedes aegypti. Insect Mol Biol 5:261–268
Muller PY, Studer E, Miserez AR (2001) Molecular BioComuting Suite: a word processor add-in for the analysis and manipulation of nucleic acid and protein sequence data. Biotechniques 31:1306–1313
Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, Lemos FJA (2002) Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem Mol Biol 32:517–523
Peters W (1992) Peritrophic membranes. In: Bradshaw D, Burggren W, Heller HC, Ishii S, Langer H, Neuweiler G, Randall DJ (eds) Zoophysiology, vol 130. Springer, Berlin Heidelberg New York
Rayms-Keller A, McGaw M, Oray C, Carlson JO, Beaty BJ (2000) Molecular cloning and characterization of a metal responsive Aedes aegypti intestinal mucin cDNA. Insect Mol Biol 9:419–426
Richards AG, Richards PA (1977) The peritrophic membranes of insects. Annu Rev Entomol 22:219–240
Schorderet S, Pearson RD, Vuocolo T, Eisemann C, Riding GA, Tellam RL (1998) cDNA and deduced amino acid sequences of a peritrophic membrane glycoprotein, “peritrophin-48”, from the larvae of Lucilia cuprina. Insect Biochem Mol Biol 28:99–111
Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC (1993) Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA 90:4266–4270
Shahabuddin M, Kaidoh T, Aikawa M, Kaslow DC (1995) Plasmodium gallinaceum: mosquito peritrophic matrix and the parasite-vector compatibility. Exp Parasitol 81:386–393
Shen Z, Jacobs-Lorena M (1998) A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin cloning, expression, and characterization. J Biol Chem 273:17665–17670
Shen Z, Jacobs-Lorena M (1999) Evolution and chitin-binding proteins in invertebrates. J Mol Evol 48:341–347
Shen Z, Dimopoulos G, Kafatos FC, Jacobs-Lorena M (1999) A cell surface mucin specifically expressed in the midgut of the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci USA 96:5610–5615
Sieber K-P, Huber M, Kaslow D, Banks SM, Torii M, Aikawa M, Miller LH (1991) The pertrophic membrane as a barrier: its penetration by Plasmodium gallinaceum and the effect of a monoclonal antibody to ookinetes. Exp Parasitol 72:145–156
Stäubli W, Freyvogel TA, Suter J (1966) Structural modifications of the endoplasmatic reticulum of midgut epithelial cells of mosquitoes in relation to blood intake. J Microsc 5:189–204
Tellam RL (1996) The peritrophic matrix In: Lehane MJ, Billingsley PF (eds) The insect midgut. Chapman & Hall, London, pp 86–114
Tellam RL, Wijffels G, Willadsen P (1999) Peritrophic matrix proteins. Insect Biochem Mol Biol 29:87–101
Terra WR (2001) The origins and functions of the insect peritrophic membrane and peritrophic gel. Arch Insect Biochem Physiol 47:47–61
Van Klinken BJ, Dekker J, Buller HA, Einerhand AWC (1995) Mucin gene structure and expression: protection versus adhesion. Am J Physiol 269:G613–G627
Van den Steen P, Rudd PM, Dwek RA, Opdenakker G (1998) Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 33:151–208
Wang P, Granados RR (1997) An intestinal mucin is the target substrate for a baculovirus enhancing. Proc Natl Acad Sci USA 94:6977–6982
Wang P, Granados RR (2001) Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch Insect Biochem Physiol 47:110–118
Wigglesworth VB (1930) The formation of the peritrophic membrane in insects, with special reference to the larvae of mosquitoes. Q J Microsc Sci 73:593–616
Wijffels G, Eisemann C, Riding G, Pearson R, Jones A, Willadsen P, Tellam R (2001) A novel family of chitin-binding proteins from insect type 2 peritrophic matrix. J Biol Chem 276:15527–15536
Zimoch L, Merzendorfer H (2002) Immunolocalization of chitin synthase in the tobacco hornworm. Cell Tissue Res 308:287–297
Acknowledgements
We thank Minh Lam of the Case Western Reserve University, Ireland Comprehensive Cancer Center, Confocal Microscopy Facility for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the National Institutes of Health. Confocal microscopy research was supported by CWRU Ireland Comprehensive Cancer Center departmental grant P30 CA43703-12.
Rights and permissions
About this article
Cite this article
Devenport, M., Fujioka, H., Donnelly-Doman, M. et al. Storage and secretion of Ag-Aper14, a novel peritrophic matrix protein, and Ag-Muc1 from the mosquito Anopheles gambiae. Cell Tissue Res 320, 175–185 (2005). https://doi.org/10.1007/s00441-004-1067-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1067-3