Abstract
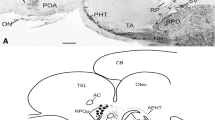
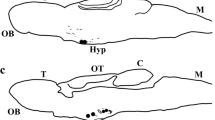
α-Melanocyte-stimulating hormone (α-MSH) is a pituitary hormone derived by post-translational processing from proopiomelanocortin and is involved in background adaptation in teleost fish. It has also been reported to suppress food intake in mammals. Here, we examined the immunocytochemical localization of α-MSH in the brain and pituitary of a pleuronectiform fish, the barfin flounder (Verasper moseri), as a first step in unraveling the possible function of α-MSH in the brain. The ontogenic development of the α-MSH system was also studied. In the pituitary, α-MSH-immunoreactive (ir) cells were preferentially detected in the pars intermedia. In the brain, α-MSH-ir neuronal somata were located in the nucleus tuberis lateralis of the basal hypothalamus, and α-MSH-ir fibers were located mainly in the telencephalon, hypothalamus, and midbrain. α-MSH-ir neuronal somata did not project their axons to the pituitary. The α-MSH-ir neurons differed from those immunoreactive to melanin-concentrating hormone. α-MSH cells in the pituitary and α-MSH-ir neuronal somata in the brain were first detected 1 day and 5 days after hatching, respectively. The distribution of α-MSH-ir cells, neuronal somata, and fibers showed a pattern similar to that in adult fish 30 days after hatching. These results indicate that the functions of α-MSH in the brain and pituitary are different and that α-MSH plays physiological roles in the early development of the barfin flounder.







Similar content being viewed by others
Abbreviations
- Dc:
-
Central part of area dorsalis telencephali
- Dd:
-
Dorsal part of area dorsalis telencephali
- Dm3:
-
Medial part of area dorsalis telencephali subdivision 3
- Dp:
-
Posterior part of area dorsalis telencephali
- HCo:
-
Horizontal commissure
- NAPv:
-
Nucleus anterior periventricularis
- NDLI:
-
Nucleus diffusus lobi inferioris
- NGp:
-
Nucleus glomerulosus pars posterior
- NH:
-
Habenula
- NLTi:
-
Nucleus lateralis tuberis pars inferior
- NLTp:
-
Nucleus lateralis tuberis pars posterior
- nMLF:
-
Nucleus of the medial longitudinal fasciculus
- NPG:
-
Nucleus preglomerulosus
- NPGc:
-
Nucleus preglomerulosus pars commissuralis
- NPGl:
-
Nucleus preglomerulosus lateralis
- NPOm:
-
Nucleus preopticus pars magnocellularis
- NPP:
-
Nucleus preopticus periventricularis
- NRLl:
-
Nucleus recessus lateralis pars lateralis
- OC:
-
Optic chiasm
- OT:
-
Optic tectum
- Pc:
-
Nucleus pretectalis centralis
- PIT:
-
Pituitary
- SPV:
-
Stratum periventriculare
- SV:
-
Saccus vasculosus
- Vc:
-
Commissural part of area ventralis telencephali
- Vp:
-
Postcommissural part of area ventralis telencephali
References
Amano M, Takahashi A, Oka Y, Yamanome T, Kawauchi H, Yamamori K (2003) Immunocytochemical localization and ontogenic development of melanin-concentrating hormone in the brain of a pleuronectiform fish, the barfin flounder. Cell Tissue Res 311:71–77
Aritaki M, Suzuki S, Watanabe K (2000) Morphological development and growth of laboratory-reared barfin flounder Verasper moseri. Nippon Suisan Gakkaishi 66:446–453
Baker BI (1991) Melanin-concentrating hormone: a general vertebrate neuropeptide. Int Rev Cytol 126:1–47
Boujard T, Leatherland JF (1992) Demand-feeding behaviour and diet pattern of feeding activity in Oncorhynchus mykiss held under different photoperiod regimes. J Fish Biol 40:535–544
Cedrá-Reverter JM, Schiöth HB, Peter RE (2003) The central melanocortin system regulates food intake in goldfish. Regul Pept 115:101–113
Dores RM, Lancha A, Rand-Weaver M, Jankelow L, Adamczyk DL (1991) Detection of a novel sequence change in the major form of alpha-MSH isolated from the intermediate pituitary of the reptile, Anolis carolinensis. Peptides 12:1261–1266
Eberle AN (1988) The melanotropins. Chemistry, physiology and mechanism of action. Karger, Basel
Kaneko T, Kakizawa S, Yada T (1993) Pituitary of “cobalt” variant of the rainbow trout separated from the hypothalamus lacks most pars intermedial and neurohypophysial tissue. Gen Comp Endocrinol 92:31–40
Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI (1983) Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature 305:321–323
Lowry PJ, Bennett HP, McMartin C (1974) The isolation and amino acid sequence of an adrenocorticotrophin from the pars distalis and a corticotrophin-like intermediate-lobe peptide from the neurointermediate lobe of the pituitary of the dogfish Squalus acanthias. Biochem J 141:427–437
Nahon JL (1994) The melanin-concentrating hormone: from the peptide to the gene. Crit Rev Neurobiol 8:221–262
Naito N, Kawazoe I, Nakai Y, Kawauchi H, Hirano T (1986) Coexistence of immunoreactivity for melanin-concentrating hormone and α-melanocyte stimulating hormone in the hypothalamus of the rat. Neurosci Lett 70:81–85
Naito N, De Jesus EG, Nakai Y, Hirano T (1993) Ontogeny of pituitary cell-types and the hypothalamo-hypophysial relationship during early development of chum salmon, Oncorhynchus keta. Cell Tissue Res 272:429–437
Oordt PGWJ van, Peute J (1983) The cellular origin of pituitary gonadotropins in teleosts. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology, vol IX, part A. Academic Press, New York, pp 137–186
Pandolfi M, Cánepa MM, Ravaglia MA, Maggese MC, Paz DA, Vissio PG (2003) Melanin-concentrating hormone system in the brain and skin of the cichlid fish Cichlasoma dimerus: anatomical localization, ontogeny and distribution in comparison to α-melanocyte-stimulating hormone-expressing cells. Cell Tissue Res 311:61–69
Rance TA, Baker BI (1979) The teleost melanin-concentrating hormone—a pituitary hormone of hypothalamic origin. Gen Comp Endocrinol 37:64–73
Rodrigues K, Sumpter JP (1983) The distribution of some proopiomelanocortin-related peptides in the pituitary gland of the rainbow trout, Salmo gairdneri. Gen Comp Endocrinol 51:454–459
Rodríguez-Gómez FJ, Rendón MC, Sarasquete C, Muñoz-Queto JA (1999) Distribution of gonadotropin releasing hormone immunoreactive systems in the brain of the Senegalese sole, Solea senegalensis. Histochem J 31:695–703
Saga T, Yamaki K, Doi Y, Yoshizuka M (1999) Chronological study of the appearance of adenohypophysial cells in the ayu (Plecoglossus altivelis). Anat Embryol 200:469–475
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Skofitsch G, Jacobowitz DM, Zamir N (1985) Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull 15:635–649
Stouthart AJ, Lucassen EC, Strien FJ van, Balm PH, Lock RA, Wendelaar Bonga SE (1998) Stress responsiveness of the pituitary-interrenal axis during early life stages of common carp (Cyprinus carpio). J Endocrinol 157:127–137
Takahashi A, Takasaka T, Yasuda A, Amemiya Y, Sakai M, Kawauchi H (2000) Identification of carp proopiomelanocortin-related peptides and their effects on phagocytes. Fish Shellfish Immunol 10:273–284
Takahashi A, Amemiya Y, Yasuda A, Meguro H, Kawauchi H (2002) Mass spectrometric detection of proopiomelanocortin (POMC)-related peptides following molecular cloning of POMC cDNA in bigeye tuna, Thunnus obesus. Fish Sci 68:1071–1078
Takahashi A, Tsuchiya K, Yamanome T, Amano M, Yasuda A, Yamamori K, Kawauchi H (2004) Possible involvement of melanin-concentrating hormone in food intake in a teleost, barfin flounder. Peptides 25:1613–1622
Yada T, Azuma T, Takahashi A, Suzuki Y, Hirose S (2000) Effects of desacetyl-α-MSH on lipid mobilization in the rainbow trout, Oncorhynchus mykiss. Zool Sci 17:1123–1127
Yada T, Moriyama S, Suzuki Y, Azuma T, Takahashi A, Hirose S, Naito N (2002) Relationship between obesity and metabolic hormones in the “cobalt” variant of rainbow trout. Gen Comp Endocrinol 128:36–43
Acknowledgements
The authors thank Ms. Kuniko Takai and Ms. Risa Ishizuka, School of Fisheries Sciences, Kitasato University, for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part by grants from the Regional Science Promotion Program Evolutional Research of the Japan Science and Technology Corporation, and the “Yumekendo-Iwate” Research and Promotion Project of the Iwate Prefectural Government Science and Technology Division to A.T.
Rights and permissions
About this article
Cite this article
Amano, M., Takahashi, A., Yamanome, T. et al. Immunocytochemical localization and ontogenic development of α-melanocyte-stimulating hormone (α-MSH) in the brain of a pleuronectiform fish, barfin flounder. Cell Tissue Res 320, 127–134 (2005). https://doi.org/10.1007/s00441-004-1058-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1058-4