Abstract
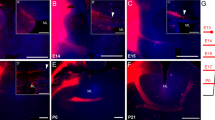
Reissner’s fiber (RF) is a threadlike structure present in the third and fourth ventricles and in the central canal of the spinal cord. RF develops by the assembly of glycoproteins released into the cerebrospinal fluid (CSF) by the subcommissural organ (SCO). SCO cells differentiate early during embryonic development. In chick embryos, the release into the CSF starts at embryonic day 7 (E7). However, RF does not form until E11, suggesting that a factor other than release is required for RF formation. The aim of the present investigation was to establish whether the factor(s) triggering RF formation is (are) intrinsic or extrinsic to the SCO itself. For this purpose, SCO explants from E13 chick embryos (a stage at which RF has formed) were grafted at two different developmental stages. After grafting, host embryos were allowed to survive for 6–7 days, reaching E9 (group 1) and E13 (group 2). In experimental group 1, the secretion released by the grafted SCOs never formed a RF; instead, it aggregated as a flocculent material. In experimental group 2, grafted SCO explants were able to develop an RF-like structure, similar to a control RF. These results suggest that the factor triggering RF formation is not present in the SCO itself, since E13 SCO secretion forms an RF in E13 brains but never develops RF-like structures when placed in earlier developmental environments. Furthermore, the glycoproteins released by implanted SCOs bind specifically to several structures: the apical portion of the mesencephalic floor plate and the choroid plexus of the third and fourth ventricles.





Similar content being viewed by others
References
Castaneyra-Perdomo A, Meyer G, Ferres-Torres R (1983) Development of the subcommissural organ in the albino mouse (a Golgi study). J Hirnforsch 24:363–370
Chouaf L, Didier-Bazes M, Hardin H, Aguera M, Fevre-Montange M, Voutsinos B, Belin MF (1991) Developmental expression of glial markers in ependymocytes of the rat subcommissural organ: role of the environment. Cell Tissue Res 266:553–561
Cifuentes M, Rodriguez EM, Hernandez S, Perez J, Peruzzo B, Perez-Figares JM, Fernandez-Llebrez P (1995) Antibody-mediated lysis of the bovine subcommissural organ maintained in culture. Exp Brain Res 107:9–51
Brio MA del, Riera P, Munoz RI, Montecinos H, Rodriguez EM (2000) The metencephalic floor plate of chick embryos expresses two secretory glycoproteins homologous with the two glycoproteins secreted by the subcommissural organ. Histochem Cell Biol 113:415–426
Brio MA del, Riera P, Peruzzo B, Rodriguez EM (2001) Hindbrain floor plate of the rat: ultrastructural changes occurring during development. Microsc Res Tech 52:615–626
Fernandez-Llebrez P, Lopez-Avalos MD, Mota MD, Cifuentes M, Andrades JA, Grondona JM, Perez J, Perez-Figares JM, Rodriguez EM (1996) Secretory glycoproteins of the roof and floor plates and their rostral derivatives, the subcommissural and flexural organs, in the developing central nervous system of vertebrates. An immunocytochemical study. Int J Dev Biol Suppl 1:151S-152S
Gato A, Martin P, Alonso MI, Martin C, Pulgar MA, Moro JA (2004) Analysis of cerebro-spinal fluid protein composition in early developmental stages in chick embryos. J Exp Zool A Comp Exp Biol 301:280–289
Grondona JM, Perez-Martin M, Cifuentes M, Perez J, Estivill-Torrus G, Perez-Figares JM, Fernandez-Llebrez P, Rodriguez EM (1998) Neuraminidase injected into the cerebrospinal fluid impairs the assembly of the glycoproteins secreted by the subcommissural organ preventing the formation of Reissner’s fiber. Histochem Cell Biol 109:391–398
Guinazu MF, Richter HG, Rodriguez EM (2002) Bovine floor plate explants secrete SCO-spondin. Cell Tissue Res 308:177–191
Hamburger V, Hamilton H (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–91
Irigoin C, Rodriguez EM, Heinrichs M, Frese K, Herzog S, Oksche A, Rott R (1990) Immunocytochemical study of the subcommissural organ of rats with induced postnatal hydrocephalus. Exp Brain Res 82:384–392
Karoumi A, Croisille Y, Croisille F, Meiniel R, Belin MF, Meiniel A (1990) Glycoprotein synthesis in the subcommissural organ of the chick embryo. II. An immunochemical study. J Neural Transm Gen Sect 80:203–212
Kimble JE, Mollgard K (1975) Subcommissural organ-associated neurons in fetal and neonatal rabbit. Cell Tissue Res 159:195–204
Lehmann W, Sterba G (1989) Tissue culture of rat subcommissural organ in vitro. Biomed Res 10(Suppl 30):11–18
Lehmann W, Sterba G (1993) The subcommissural organ in vitro. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 133–140
Lehmann W, Sterba G, Wobus AM (1989) Primary culture of the mouse subcomissural organ in vitro. Acta Zool 70:199–203
Lehmann W, Naumann W, Wagner U (1993) Tissue culture of bovine subcommissural organ. Anat Embryol 187:505–514
Leonhardt H (1980) Ependym und circumventrikuläre Organe. In: Oksche A, Vollrath L (eds) Neuroglia I (Handbuch der Mikroskopischen Anatomie des Menschen, vol IV, part 10). Springer, Berlin Heidelberg New York, pp 176–665
Lichtenfeld J, Viehweg J, Schützenmeister J, Naumann WW (1999) Reissner’s substance expressed as a transient pattern in vertebrate floor plate. Anat Embryol 200:161–174
Lopez-Avalos MD, Cifuentes M, Grondona JM, Miranda E, Perez J, Fernandez-Llebrez P (1997) Rostral floor plate (flexural organ) secretes glycoproteins immunologically similar to subcommissural organ glycoproteins in dogfish (Scyliorhinus canicula) embryos. Brain Res Dev Brain Res 102:69–75
Marcinkiewicz M, Bouchaud C (1983) The ependymal secretion of the fetal and adult rat subcommissural organ. Morphological aspects linked to the synthesis, storage and release of the secretory products. Biol Cell 48:47–52
Marcinkiewicz M, Bouchaud C (1986) Formation and maturation of axo-glandular synapses and concomitant changes in the target cells of the rat subcommissural organ. Biol Cell 56:57–65
Meiniel A (2001) SCO-spondin, a glycoprotein of the subcommissural organ/Reissner’s fiber complex: evidence of a potent activity on neuronal development in primary cell cultures. Microsc Res Tech 52:484–495
Meiniel R, Didier R, Molat JL, Meiniel A (1993) Developmental aspects of the subcommissural organ: an approach using lectins and monoclonal antibodies. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 51–59
Miranda E, Almonacid JA, Rodriguez S, Perez J, Hein S, Cifuentes M, Fernandez-Llebrez P, Rodriguez EM (2001) Searching for specific binding sites of the secretory glycoproteins of the subcommissural organ. Microsc Res Tech 52:541–551
Møllgard K (1972) Histochemical investigations on the human foetal subcommissural organ. I. Carbohydrates and mucosubstances, proteins and nucleoproteins, esterase, acid and alkaline phosphatase. Histochemie 32:31–48
Naumann W (1986) Immunhistochemische Untersuchungen zur Ontogenese des Subcommissuralorgans. Acta Histochem Suppl 33:265–272
Naumann W, Müller G, Kloss P (1987) Immunoreactive glycoproteins of the subcommissural organ in the embryonic stages of the vertebrate brain. Wiss Z Karl-Marx-Univ Leipzig Math-Naturwiss R 36:17–20
Nualart F, Hein S (2001) Biosynthesis and molecular biology of the secretory proteins of the subcommissural organ. Microsc Res Tech 52:468–483
Nualart F, Hein S, Rodriguez EM, Oksche O (1991) Identification and partial characterization of the secretory glycoproteins of the bovine subcommissural organ-Reissner’s fiber complex. Evidence for the existence of two precursor forms.Brain Res Mol Brain Res 11:227–238
Oksche A (1956) Funktionelle histologische Untersuchungen über die Organe des Zwischenhirndaches der Chordaten. Anat Anz 102:404–419
Oksche A (1961) Vergleichende Untersuchungen über die sekretorische Aktivität der Subkommissuralorgans und den Gliacharakter seiner Zellen. Z Zellforsch Mikrosk Anat 54:549–612
Oksche A (1969) The subcommissural organ. J Neurovisc Relat Suppl 9:111–139
Olsson R (1958) Studies on the subcommissural organ. Acta Zool 39:71–102
Olsson R (1961) Subcommissural ependyma and pineal organ development in human fetuses. Gen Comp Endocrinol 1:117–123
Richter HG, Munoz RI, Millan CS, Guinazu MF, Yulis CR, Rodriguez EM (2001) The floor plate cells from bovine express the mRNA encoding for SCO-spondin and its translation products. Brain Res Mol Brain Res 93:137–147
Rodriguez EM, Oksche A, Hein S, Rodriguez S, Yulis R (1984) Comparative immunocytochemical study of the subcommissural organ. Cell Tissue Res 237:427–441
Rodriguez EM, Rodriguez S, Schoebitz K, Yulis CR, Hoffmann P, Manns V, Oksche A (1989) Light- and electron-microscopic investigation of the rat subcommissural organ grafted under the kidney capsule, with particular reference to immunocytochemistry and lectin histochemistry. Cell Tissue Res 258:499–514
Rodriguez S, Rodriguez EM, Jara P, Peruzzo B, Oksche A (1990) Single injection into the cerebrospinal fluid of antibodies against the secretory material of the subcommissural organ reversibly blocks formation of Reissner’s fiber: immunocytochemical investigations in the rat. Exp Brain Res 81:113–124
Rodriguez EM, Oksche A, Hein S, Yulis CR (1992) Cell biology of the subcommissural organ. Int Rev Cytol 135:39–121
Rodriguez EM, Jara P, Richter H, Montecinos H, Flandez B, Wiegand R, Oksche A (1993) Evidence for the release of CSF-soluble secretory material from the subcommissural organ, with particular reference to the situation in the human. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 121–131
Rodriguez EM, Brio Leon MA del, Riera P, Menendez J, Schoebitz K (1996) The floor plate of the hindbrain is a highly specialized gland: immunocytochemical and ultrastructural characteristics. Brain Res Dev Brain Res 97:153–168
Rodriguez EM, Rodriguez S, Hein S (1998) The subcommissural organ. Microsc Res Tech 41:98–123
Rodriguez S, Navarrete EH, Vio K, Gonzalez C, Schobitz K, Rodriguez EM (1999) Isograft and xenograft of the subcommissural organ into the lateral ventricle of the rat and the formation of Reissner’s fiber. Cell Tissue Res 296:457–469
Rodriguez EM, Oksche A, Montecinos H (2001) Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech 52:573–590
Sargent PE (1900) Reissner‘s fibre in the canalis centralis of vertebrates. Anat Anz 17:33–44
Schoebitz K, Garrido O, Heinrichs M, Speer L, Rodriguez EM (1986) Ontogenical development of the chick and duck subcommissural organ: an immunocytochemical study. Histochemistry 84:1–40
Schoebitz K, Rodriguez EM, Garrido O, Brio MA del (1993) Ontogenetic development of the subcommissural organ with reference to the flexural organ. In: Oksche A, Rodriguez EM, Fernandez-Llebrez P (eds) The subcommissural organ. Springer, Berlin Heidelberg New York, pp 41–49
Schoebitz K, Gonzalez C, Peruzzo B, Yulis CR, Rodriguez EM (2001) Organ culture of the bovine subcommissural organ: evidence for synthesis and release of the secretory material. Microsc Res Tech 52:496–509
Sheffield JB, Graff D (1991) Extracellular proteases in developing chick neural retina. Exp Eye Res 52:733–741
Sterba G, Ermisch A, Freyer K, Hartmann G (1967) Incorporation of sulphur-35 into the subcommissural organ and Reissner’s fibre. Nature 216:504
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 19:4994–5004
Wingstrand KG (1953) Neurosecretion and antidiuretic activity in chick embryos with remarks on the subcommissural organ. Ark Zool 6:41–67
Wislocki GB, Roth WD (1958) Selective staining of the human subcommissural organ. Anat Rec 130:125–133
Yong VW, Power C, Forsyth P, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511
Yulis CR, Mota MD, Andrades JA, Rodriguez S, Peruzzo B, Mancera JM, Ramirez P, Garrido M, Perez-Figarez JM, Fernandez-Llebrez P, Rodriguez EM (1998) Floor plate and the subcommissural organ are the source of secretory compounds of related nature: comparative immunocytochemical study. J Comp Neurol 392:19–34
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Hoyo-Becerra and M. D. López-Avalos contributed equally to this study and should be considered as joint first authors.
C. Hoyo-Becerra was the recipient of a predoctoral fellowship (PFPI) from the Ministerio de Ciencia y Tecnología (Spain). This work was supported by grants from DGICYT (BFI2003-03348; Spain) and FIS (01/0948; Spain), FIS (01–0948, PI021517; Spain) and ISCIII (red CIEN, nodo Fundación Carlos Haya).
Rights and permissions
About this article
Cite this article
Hoyo-Becerra, C., López-Avalos, M.D., Alcaide-Gavilán, M. et al. Reissner’s fiber formation depends on developmentally regulated factors extrinsic to the subcommissural organ. Cell Tissue Res 321, 429–441 (2005). https://doi.org/10.1007/s00441-004-1040-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1040-1