Abstract
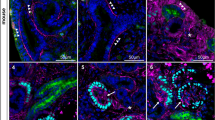
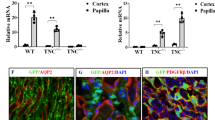
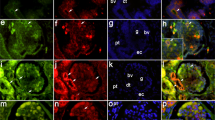
Growth of the kidney is a complex process piloted by the collecting duct (CD) ampullae. The dichotomous arborisation and consecutive elongation of this tubular element determines the exact site and time for the induction of nephrons in the overlaying mesenchymal cap condensates. The mechanism by which the CD ampullae find the correct orientation is currently unknown. Recently, we have demonstrated micro-fibres that originate from the basal aspect of the CD ampullae and extend through the mesenchyme to the organ capsule. The micro-fibres are assumed to be involved in the growth and arborisation process of the CD ampulla. Therefore, we have investigated the specific distribution of the micro-fibres during branching morphogenesis. We have also analysed whether the micro-fibres co-localise with extracellular matrix (ECM)-modulating enzymes and whether the co-localisation pattern changes during CD ampulla arborisation. Micro-fibres were detected in all stages of CD ampulla arborisation. Tissue transglutaminase (Tgase2) co-localised with soybean agglutinin (SBA)-positive micro-fibres, whose presence depended upon the degree of CD branching. Matrix metalloproteinase-9 (MMP-9) also co-localised with micro-fibres, but its expression pattern was different from that for Tgase2. Western blotting experiments demonstrated that Tgase2 and MMP-9 co-migrated with SBA-labelled proteins. Thus, the micro-fibres are developmentally modulated by enzymes of the ECM in embryonic kidney cortex. These findings illustrate the importance of micro-fibres in directing CD ampulla growth.







Similar content being viewed by others
References
Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99:377–386
Bard JB, Gordon A, Sharp L, Sellers WI (2001) Early nephron formation in the developing mouse kidney. J Anat 199:385–392
Davies J, Lyon M, Gallagher J, Garrod D (1995) Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development 121:1507–1517
Dekel B, Reisner Y (2004) Embryonic committed stem cells as a solution to kidney donor shortage. Expert Opin Biol Ther 4:443–454
Douthwaite JA, Johnson TS, Haylor JL, Watson P, El Nahas AM (1999) Effects of transforming growth factor-beta1 on renal extracellular matrix components and their regulating proteins. J Am Soc Nephrol 10:2109–2119
Fisher CE, Michael L, Barnett MW, Davies JA, Davies J, Lyon M, Gallagher J, Garrod D (2001) Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney: sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development 128:4329–4338
Kim SY, Jeong EJ, Steinert PM (2002) IFN-gamma induces transglutaminase 2 expression in rat small intestinal cells. J Interferon Cytokine Res 22:677–682
Legallicier B, Trugnan G, Murphy G, Lelongt B, Ronco P (2001) Expression of the type IV collagenase system during mouse kidney development and tubule segmentation. J Am Soc Nephrol 12:2358–2369
Martinez J, Rich E, Barsigian C (1989) Transglutaminase-mediated cross-linking of fibrinogen by human umbilical vein endothelial cells. J Biol Chem 264:20502–20508
Minuth WW (1987) Neonatal rabbit kidney cortex in culture as tool for the study of collecting duct formation and nephron differentiation. Differentiation 36:12–22
Mohan K, Pinto D, Issekutz TB (2003) Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol 171:3179–3186
Ohtsuka T, Ota M, Nio N, Motoki M (2000) Comparison of substrate specifities of transglutaminase using synthetic peptides as acyl donors. Biosci Biotechnol Biochem 64:2608–2613
Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO (2001) TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128:1045–1057
Ritvos O, Tuuri T, Eramaa M, Sainio K, Hilden K, Saxen L, Gilbert SF (1995) Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech Dev 50:229–245
Sakai T, Larsen M, Yamada KM (2003) Fibronectin requirement in branching morphogenesis. Nature 423:876–881
Sariola H (2002) Nephron induction revisited: from caps to condensates. Curr Opin Nephrol Hypertens 11:17–21
Saxen L (1987) Organogenesis of the kidney. Cambridge University Press, Cambridge
Schumacher K, Strehl R, De Vries U, Groene HJ, Minuth WW (2002a) SBA-positive fibers between the CD ampulla, mesenchyme, and renal capsule. J Am Soc Nephrol 13:2446–2453
Schumacher K, Strehl R, Minuth WW (2002b) Detection of glycosylated sites in embryonic rabbit kidney by lectin chemistry. Histochem Cell Biol 118:79–87
Schumacher K, Strehl R, Minuth WW (2003) Characterization of micro-fibers at the interface between the renal collecting duct ampulla and the cap condensate. Nephron Exp Nephrol 95:e43–e54
Singh US, Kunar MT, Kao YL, Baker KM (2001) Role of transglutaminase II in retinoic acid-induced activation of RhoA-associated kinase-2. Embo J 20:2413–2423
Smyth BJ, Snyder RW, Balkovetz DF, Lipschutz JH (2003) Recent advances in the cell biology of polycystic kidney disease. Int Rev Cytol 231:51–89
Strehl R, Minuth WW (2001a) Partial identification of the mab (CD)Amp1 antigen at the epithelial-mesenchymal interface in the developing kidney. Histochem Cell Biol 116:389–396
Strehl R, Minuth WW (2001b) Nephron induction–the epithelial mesenchymal interface revisited. Pediatr Nephrol 16:38–40
Strehl R, Kloth S, Aigner J, Steiner P, Minuth WW (1997) PCDAmp1, a new antigen at the interface of the embryonic collecting duct epithelium and the nephrogenic mesenchyme. Kidney Int 52:1469–1477
Tanney DC, Feng L, Pollock AS, Lovett DH (1998) Regulated expression of matrix metalloproteinases and TIMP in nephrogenesis. Dev Dyn 213:121–129
Tezuka K, Nemoto K, Tezuka Y, Sato T, Ikeda Y, Kobori M, Kawashima H, Eguchi H, Hakeda Y, Kumegawa M (1994) Identification of matrix metalloproteinase 9 in rabbit osteoclasts. J Biol Chem 269:15006–15009
Vainio S, Lehtonen E, Jalkanen M, Bernfield M, Saxen L (1989) Epithelial-mesenchymal interactions regulate the stage-specific expression of a cell surface proteoglycan, syndecan, in the developing kidney. Dev Biol 134:382–391
Verderio EA, Telci D, Okoye A, Melino G, Griffin M (2003) A novel RGD-independent cell adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem 278:42604–42614
Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G (2004) PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci USA 101:2311–2316
Acknowledgements
The technical help of Mrs. L. Denk and Mrs. S. Lukas is gratefully acknowledged. We thank Dr. Hayo Castrop for critical comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schumacher, K., Klar, J., Wagner, C. et al. Temporal-spatial co-localisation of tissue transglutaminase (Tgase2) and matrix metalloproteinase-9 (MMP-9) with SBA-positive micro-fibres in the embryonic kidney cortex. Cell Tissue Res 319, 491–500 (2005). https://doi.org/10.1007/s00441-004-1028-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1028-x