Abstract
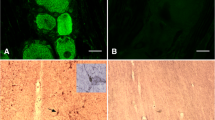
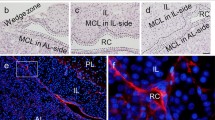
Increasing evidence suggests that multihormonal cells in the pituitary gland may be more commonplace than previously thought. This has forced us to reconsider our classical view of cell populations in the pituitary gland. Studies so far have focused almost exclusively on the rat, and there is a dearth of information on other species. Our first objective was to determine whether a subpopulation of gonadotropes also express somatotropin in the ewe, as reported in the rat. In addition, we sought to determine whether gonadotropes express any of the other known pituitary hormones. Finally, we investigated whether the stage of the estrous cycle influenced the occurrence of these pluripotential gonadotropes. We found that a small population of βLH-immunoreactive cells also expresses immunoreactive GH, prolactin and TSH. No gonadotropes colocalized with ACTH. Significantly (P<0.001) more gonadotropes expressed GH during the luteal (10.7±0.4%) than the late follicular (5.4±0.3%) phase but there was no difference between the luteal and follicular phases in the proportion of gonadotropes expressing prolactin (follicular: 5.7±0.7%; luteal: 5.5±0.6%) or TSH (follicular: 3.1±0.7%; luteal: 4.2±0.5%). Similarly, there was a significant (P<0.05) difference in the proportion of GH-immunoreactive cells expressing βLH immunoreactivity in the luteal (5.9±0.3%) and follicular (3.4±0.5%) phases but no difference in the proportion of prolactin- (follicular: 2.2±0.7%; luteal: 2.0±0.8%) or TSH-immunoreactive cells (follicular: 9.6±3.7%; luteal: 10.8±2.9%) expressing βLH. The specific function of these multihormonal gonadotropes in sheep remains to be determined.




Similar content being viewed by others
References
Amar AP, Weiss MH (2003) Pituitary anatomy and physiology. Neurosurg Clin N Am 14:11–23
Anderson ST, Walsh JP, Tillet Y, Clarke IJ, Curlewis JD (2001) Dopaminergic input to the ventromedial hypothalamus facilitates the oestrogen-induced luteinizing hormone surge in ewes. Neuroendocrinology 73:91–101
Arbogast LA, Ben-Jonathan N (1988) The preovulatory prolactin surge: an evaluation of the role of dopamine. Endocrinology 123:2690–2695
Bachelot A, Monget P, Imbert-Bollore P, Coshigano K, Kopchick JJ, Kelly PA, Binart N (2002) Growth hormone is required for ovarian follicular growth. Endocrinology 143:4104–4112
Bevers MM, Izadyar F (2002) Role of growth hormone and growth hormone receptor in oocyte maturation. Mol Cell Endocrinol 197:173–178
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA (1998) Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 19:225–268
Bowen JM, Keyes PL (1999) The proestrous prolactin surge is not the sole initiator of regressive changes in corpora lutea of normally cycling rats. Biol Reprod 61:1208–1215
Bowen JM, Telleria CM, Towns R, Keyes PL (2000) Downregulation of long-form prolactin receptor mRNA during prolactin-induced luteal regression. Eur J Endocrinol 143:285–292
Cabell SB, Esbenshade KL (1990) Effect of feeding thyrotropin-releasing hormone to lactating sows. J Anim Sci 68:4292–4302
Campbell B, Mann G, McNeilly A, Baird D (1990) The pattern of ovarian inhibin, estradiol, and androstenedione secretion during the estrous cycle of the ewe. Endocrinology 127:227–235
Childs GV (1991) Multipotential pituitary cells that contain adrenocorticotropin (ACTH) and other pituitary hormones. Trends Endocrinol Metab 2:112–117
Childs GV (1997) Cytochemical studies of multifunctional gonadotropes. Microsc Res Tech 39:114–130
Childs GV (2002) Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem 110:42–49
Childs GV, Ellison DG, Garner LL (1980) An immunocytochemist’s view of gonadotropin storage in the adult male rat: cytochemical and morphological heterogeneity in serially sectioned gonadotropes. Am J Anat 158:397–409
Childs GV, Ellison DG, Ramaley JA (1982) Storage of anterior lobe adrenocorticotropin in corticotropes and a subpopulation of gonadotropes during the stress-nonresponsive period in the neonatal male rat. Endocrinology 110:1676–1692
Childs GV, Unabia G, Miller BT (1994a) Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology 134:1943–1951
Childs GV, Unabia G, Rougeau D (1994b) Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) beta-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH beta, FSH beta, and/or growth hormone. Endocrinology 134:990–997
Childs GV, Miller BT, Miller WL (1997) Differential effects of inhibin on gonadotropin stores and gonadotropin-releasing hormone binding to pituitary cells from cycling female rats. Endocrinology 138:1577–1584
Childs GV, Unabia G, Miller BT, Collins TJ (1999) Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol 162:177–187
Childs GV, Unabia G, Wu P (2000) Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology 141:1560–1570
Codner E, Cassorla F (2002) Growth hormone and reproductive function. Mol Cell Endocrinol 186:133–136
De Moraes GV, Vera-Avila HR, Lewis AW, Koch JW, Neuendorff DA, Hallford DM, Reeves JJ, Randel RD (1998) Influence of hypo- or hyperthyroidism on ovarian function in Brahman cows. J Anim Sci 76:871–879
Djahanbakhch O, McNeilly AS, Warner PM, Swanston IA, Baird DT (1984) Changes in plasma levels of prolactin, in relation to those of FSH, oestradiol, androstenedione and progesterone around the preovulatory surge of LH in women. Endocrinology 110:1292–1299
Duval DL, Farris AR, Quirk CC, Nett TM, Hamernik DL, Clay CM (2000) Responsiveness of the ovine gonadotropin-releasing hormone receptor gene to estradiol and gonadotropin-releasing hormone is not detectable in vitro but is revealed in transgenic mice. Endocrinology 141:1001–1010
Eagle RC, Tortonese DJ (2000) Characterization and distribution of gonadotrophs in the pars distalis and pars tuberalis of the equine pituitary gland during the estrous cycle and seasonal anestrus. Biol Reprod 63:826–832
Faria ACS, Bekenstein LW, Booth RA, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS (1992) Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol 36:591–596
Gross DS, Page RB (1979) Luteinizing hormone and follicle-stimulating hormone production in the pars tuberalis of hypophysectomised rats. Am J Anat 156:285–291
Homburg R, West C, Torresani T, Jacobs HS (1990) Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertil Steril 53:254–260
Homburg R, Levy T, Ben-Rafael Z (1995) Adjuvant growth hormone for induction of ovulation with gonadotrophin-releasing hormone agonist and gonadotrophins in polycystic ovary syndrome: a randomized, double-blind, placebo controlled trial. Hum Reprod 10:2550–2553
Honda J, Manabe Y, Matsumura R, Takeuchi S, Takahashi S (2003) IGF-I regulates pro-opiomelanocortin and GH gene expression in the mouse pituitary gland. J Endocrinol 178:71–82
Howes K, Menendez-Pelaez A, Reiter R, Vaughan M, Hensel C, Vaughan G (1991) Serum luteinizing hormone, prolactin, and thyrotropin and their pituitary subunit mRNA levels during proestrus in the Syrian hamster. Neuroendocrinology 54:629–634
Hull KL, Harvey S (2001) Growth hormone: roles in female reproduction. J Endocrinol 168:1–23
Landefeld TD, Suttie JM (1989) Changes in messenger ribonucleic acid concentrations and plasma levels of growth hormone during the ovine estrous cycle and in response to exogenous estradiol. Endocrinology 125:1474–1478
Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG (1990) Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533
Losinski NE, Horvath E, Kovacs K (1989) Double-labeling immunogold electron-microscopic study of hormonal colocalization in nontumorous and adenomatous rat pituitaries. Am J Anat 185:236–243
Lucy MC, Bilby CR, Kirby CJ, Yuan W, Boyd CK (1999) Role of growth hormone in development and maintenance of follicles and corpora lutea. J Reprod Fertil Suppl 54:49–59
Malven PV, Haglof SA, Jiang H (1995) Serum concentrations of luteinizing hormone, growth hormone, and prolactin in untreated and estradiol-treated ovariectomized ewes after immunoneutralization of hypothalamic neuropeptide Y. J Anim Sci 73:2105–2112
Mattheij JA, Swarts JJ, Lokerse P, van Kampen JT, Van der Heide D (1995) Effect of hypothyroidism on the pituitary-gonadal axis in the adult female rat. J Endocrinol 146:87–94
Meeran D, Urbanski HF, Gregory SJ, Townsend J, Tortonese DJ (2003) Developmental changes in the hormonal identity of gonadotroph cells in the rhesus monkey pituitary gland. J Clin Endocrinol Metab 88:2934–2942
Moriarty GC, Garner LL (1977) Immunocytochemical studies of cells in the rat adenohypophysis containing both ACTH and FSH. Nature 265:356–358
Newman GR, Jasani B, Williams ED (1989) Multiple hormone storage by cells of the human pituitary. J Histochem Cytochem 37:1183–1192
Nunez L, Villalobos C, Senovilla L, Garcia-Sancho J (2003) Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J Physiol 549:835–843
Okada Y, Fujii Y, Moore JP Jr, Winters SJ (2003) Androgen receptors in gonadotrophs in pituitary cultures from adult male monkeys and rats. Endocrinology 144:267–273
Parhar IS, Soga T, Sakuma Y, Millar RP (2002) Spatio-temporal expression of gonadotropin-releasing hormone receptor subtypes in gonadotropes, somatotropes and lactotropes in the cichlid fish. J Neuroendocrinol 14:657–665
Rillema JA (1980) Mechanism of prolactin action. Fed Proc 39:2593–2598
Salle A, Klein M, Pascal-Vigneron V, Dousset B, Leclere J, Weryha G (2000) Successful pregnancy and birth after sequential cotreatment with growth hormone and gonadotropins in a woman with panhypopituitarism: a new treatment protocol. Fertil Steril 74:1248–1250
Scanlan N, Skinner DC (2002) Estradiol modulation of growth hormone secretion in the ewe: no growth hormone-releasing hormone neurons and few somatotropes express estradiol receptor α. Biol Reprod 66:1267–1273
Shoham Z, Homburg R, Owen EJ, Conway GS, Ostergaard H, Jacobs HS (1992) The role of treatment with growth hormone in infertile patients. Bailliere’s Clin Obstet Gynaecol 6:267–281
Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW (1990) Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4:695–711
Skinner DC, Caraty A (2003) Prolactin release during the estradiol-induced LH surge in ewes: modulation by progesterone but no evidence for prolactin-releasing peptide involvement. J Endocrinol 177:453–460
Skinner DC, Robinson JE (1995) Melatonin-binding sites in the gonadotroph-enriched zona tuberalis of ewes. J Reprod Fertil 104:243–250
Skinner DC, Robinson JE (1996) The pars tuberalis of the ewe: no effect of season or ovariectomy on the distribution, density or presence of immunoreactive cells. Cell Tissue Res 284:117–123
Stefano AV, Vissio PG, Paz DA, Somoza GM, Maggese MC, Barrantes GE (1999) Colocalization of GnRH binding sites with gonadotropin-, somatotropin-, somatolactin-, and prolactin-expressing pituitary cells of the pejerrey, Odontesthes bonariensis, in vitro. Gen Comp Endocrinol 116:133–139
Thomas SG, Clarke IJ (1997) The positive feedback action of estrogen mobilizes LH-containing, but not FSH-containing secretory granules in ovine gonadotropes. Endocrinology 138:1347–1350
Tixier-Vidal A, Tougard C, Kerdelhue B, Jutisz M (1975) Light and electron microscopic studies on immunocytochemical localization of gonadotropic hormones in the rat pituitary gland with antisera against ovine FSH, LH, LHα, and LHβ. Ann N Y Acad Sci 254:433–461
Tobin VA, Pompolo S, Clarke IJ (2001) The percentage of pituitary gonadotropes with immunoreactive oestradiol receptors increases in the follicular phase of the ovine oestrous cycle. J Neuroendocrinol 13:846–854
Vidal S, Roman A, Oliveira MC, De La Cruz LF, Moya L (1998) Simultaneous localization of Pit-1 protein and gonadotropins on the same cell type in the anterior pituitary glands of the rat. Histochem Cell Biol 110:183–188
Villalobos C, Nunez L, Garcia-Sancho J (2004) Anterior pituitary thyrotropes are multifunctional cells. Am J Physiol Endocrinol Metab 287:E1166–1170
Yonezawa T, Mogi K, Li JY, Sawasaki T, Suzuki M, Yamanouchi K, Nishihara M (2003) Changes in the pulsatile pattern of growth hormone secretion during the estrous cycle in goats. Ann Meeting Soc Neurosci, New Orleans (Abstract 924.914)
Acknowledgements
The polyclonal rabbit anti-βLH antibody and the polyclonal guinea-pig anti-TSH antibody were kindly provided by Dr. A.F. Parlow, NIDDK.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by NIH grant (5 P20 RR15553).
Rights and permissions
About this article
Cite this article
Mignot, M., Skinner, D.C. Colocalization of GH, TSH and prolactin, but not ACTH, with βLH-immunoreactivity: evidence for pluripotential cells in the ovine pituitary. Cell Tissue Res 319, 413–421 (2005). https://doi.org/10.1007/s00441-004-1009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1009-0