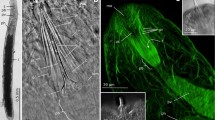
Abstract
The tubiform Dufour gland in the digger wasp species Liris niger is about 1.0 mm long (∅ 0.15 mm). An alternating arrangement of longitudinal and circumferential bundles of striated muscle fibers surrounds the gland. The Dufour gland, together with the venom gland, enters the sting base and terminates in the sting. The glandular epithelium is monolayered. Glands about 3 day after imaginal ecdysis have an empty lumen but a thick lining epithelium. The gland cells are characterized by a well-developed vesicular smooth endoplasmic reticulum, sparse rough ER and numerous free ribosomes. They also exhibit several electron-lucent vesicles and autophagic vacuoles. Secretion of electron-dense material via the gland cuticle into the gland lumen is apparent. Glands more than 20 days after imaginal ecdysis display a large lumen and a thin epithelium. The cells show signs of degeneration with numerous cytolytic inclusions. Dufour gland liquid contains numerous polypeptides of molecular weights ranging from 14 to about 200 kDa. In addition the secretion consists predominantly of straight-chain hydrocarbons, accompanied by small amounts of esters. The major hydrocarbons are pentadecane and (Z)-8-heptadecene. Dufour gland secretion may have several functions: (1) the polypeptides might be involved in the gluing process of the eggs, while (2) the hydrocarbon oils may function as lubricants for the lancets and (3) might soften the secretion, thus allowing easier application of the glue. The lipophilic volatile material (4) might also be involved in pheromonal signaling.







Similar content being viewed by others
References
Abdalla FC, Velthuis H, Cruz-Landim C da, Duchateau MJ (1999) Changes in the morphology and ultrastructure of the Dufour’s gland during the life cycle of the bumble bee queen, Bombus terrestris L. (Hymenoptera: Bombini). Netherlands J Zool 49:251–261
Ali MF, Attygalle AB, Billen JPJ, Morgan ED (1988a) Contents of Dufour glands in some formicine ants: queens and workers of Camponotus aethiops and a re-examination of Lasius fuliginosus. Entomol Exp Appl 46:109–115
Ali MF, Attygalle AB, Billen JPJ, Jackson BD, Morgan ED (1988b) Change of Dufour gland contents with age of workers of Formica sanguinea (Hymenoptera: Formicidae). Physiol Entomol 13:249–255
Altenkirch G (1962) Untersuchungen über die Morphologie der abdominalen Hautdrüsen einheimischer Apiden. Zool Beitr 7:161–238
Anton S, Gnatzy W (1998) Prey specificity and the importance of close-range chemical cues in prey recognition in the digger wasp, Liris niger. J Insect Behav 11:671–690
Attygalle AB, Billen JPJ, Jackson BD, Morgan ED (1990) Morphology and chemical contents of Dufour glands of Pseudomyrmex ants (Hymenoptera: Formicidae). Z Naturforsch 45c:691–697
Attygalle AB, Jessen K, Bestmann H-H, Buschinger A, Maschwitz U (1996) Oily substances from gastral intersegmental glands of the ant Pachycondyla tridentata (Ponerinae): lack of pheromone function in tandem running and antibiotic effects but further evidence for lubricative function. Chemoecology 7:8–12
Bart K, Farley P (1990) Ultrastructure of Dufour’s gland in Formica glacialis (Hymenoptera: Formicidae). Trans Am Microsc Soc 109:107–111
Bender JC (1943) Anatomy and histology of the female reproductive organs of Habrobracon juglandis (Ashmead) (Hym., Braconidae). Ann Entomol Soc Am 36:537–545
Bestmann HJ, Janssen E, Kern F, Liepold B (1995) All-trans geranylgeranyl acetate and geranylgeraniol, recruitment pheromone components in the Dufour gland of the Ponerine ant Ectatomma ruidum. Naturwissenschaften 82:334–336
Billen J (1985) Comparative ultrastructure of the poison and Dufour’s glands in Old and New World army ants. Actes Coll Insectes Soc 2:17–26
Billen J (1986a) Morphology and ultrastructure of the Dufour’s and venom gland in the ant, Myrmica rubra (L.) (Hymenoptera: Formicidae). Int J Insect Morphol Embryol 15:13–25
Billen JPJ (1986b) Comparative morphology and ultrastructure of the Dufour gland in ants (Hymenoptera: Formicidae). Entomol Gener 11:165–181
Billen JPJ (1987) New structural aspects of the Dufour’s and venom gland in social insects. Naturwissenschaften 74:340–341
Billen J (1990) Morphology and ultrastructure of the Dufour’s and venom gland in the ant Myrmecia gulosa (Fabr.) (Hymenoptera: Formicidae). Aust J Zool 38:305–315
Billen J, Ito F, Tsuji K, Schoeters E, Maile R, Morgan ED (2000) Structure and chemistry of the Dufour gland in Pristomyrmex ants (Hymenoptera, Formicidae). Acta Zool 81:159–166
Billen J, Grasso DA, Mori A, Le Moli F (2001) Structural and functional changes of the Dufour gland in gynes of the amazon ant Polyergus rufescens (Hymenoptera, Formicidae). Zoomorphology 121:55–61
Blass S, Ruthmann A (1989) Fine structure of the accessory gland of the female genital tract of the ichneumonid Pimpla turionellae (Insecta, Hymenoptera). Zoomorphology 108:367–377
Blomquist GJ, Nelson DR, de Renobales M (1987) Chemistry, biochemistry, and physiology of insect cuticular lipids. Arch Insect Biochem Physiol 6:227–265
Brothers DJ (1999) Phylogeny and evolution of wasps, ants and bees (Hymenoptera, Chrysidoidea, Vespoidea and Apoidea). Zool Scripta 28:233–249
Buser HR, Arn H, Guerin P, Rauscher S (1983) Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal Chem 55:818–822
Cane HJ, Brooks RW (1983) Dufour’s gland lipid chemistry of three species of Centris bees (Hymenoptera: Apoidea, Anthophoridae). Comp Biochem Physiol 76B:895–897
Cane HJ, Carlson RG (1984) Dufour’s gland triglycerides from Antophora, Emphoropsis (Anthophoridae) and Megachile (Megachilidae) bees (Hymenoptera: Apoidea). Comp Biochem Physiol 78B:769–772
Cassier P, Lensky Y (1995) Ultrastructure of the wax gland complex and the secretion of beeswax in the worker honey bee Apis mellifera L. Apidologie 26:17–26
Cruz-López L, Patricio EF, Morgan ED (2001) Secretions of stingless bees: the Dufour gland of Nannotrigona testaceicornis. J Chem Ecol 27:69–80
Dani FR, Morgan ED, Turillazzi S (1996) Dufour gland secretion of Polistes wasp: chemical composition and possible involvement in nestmate recognition (Hymenoptera: Vespidae). J Insect Physiol 42:541–548
Dufour L (1841) Recherches anatomiques et physiologiques sur les Orthoptères, les Hyménoptères et les Neuroptères. Mémoires de l’Académie des Sciences, Institute de France, vol 7, pp 265–647
D’Ettore P, Errard Ch, Ibarra F, Franke W, Hefetz A (2000) Sneak in or repel your enemy: Dufour’s gland repellent as a strategy for successful ursurpation in the slave-maker Polyergus rufescens. Chemoecology 10:135–142
Ferber M, Consoulas C, Gnatzy W (1999) Digger wasp vs. cricket: immediate actions of the predator’s paralytic venom on the CNS of the prey. J Neurobiol 38:323–337
Ferber M, Hörner M, Cepok S, Gnatzy W (2001) Digger wasp versus cricket: mechanisms underlying the total paralysis caused by the predator’s venom. J Neurobiol 47:207–222
Fling SP, Gregerson DS (1986) Peptide and protein molecular weight determination by electrophoresis using a high-molarity TRIS buffer system without urea. Anal Biochem 155:83–88
Francis GW, Veland K (1981) Alkylthiolation for the determination of double-bond positions in linear alkenes. J Chromatogr 219:379–384
Geleff S, Böck P (1984) Pancreatic duct glands. II. Lectin binding affinities of ductular epithelium, ductular glands, and Brunner glands. Histochemistry 80:31–38
Gnatzy W (1996) Digger wasp vs. cricket: neuroethology of a predator-prey interaction. In: Lindauer M (ed) Information processing in animals, vol 10. Fischer-Verlag, Stuttgart, pp 1–92
Gnatzy W, Heußlein R (1986) Digger wasp against crickets: I. receptors involved in the antipredator strategies of the prey. Naturwissenschaften 73:212–215
Gnatzy W, Otto D (1996) Digger wasp vs. cricket: application of the paralytic venom by the predator and changes in behavioural reactions of the prey after being stung. Naturwissenschaften 83:467–470
Gnatzy W, Volknandt W (2000) Venom gland of the digger wasp Liris niger: morphology, ultrastructure, age-related changes and biochemical aspects. Cell Tissue Res 302:271–284
Gökçen OA, Morgan ED, Dani FR, Agost D, Wehner R (2002) Dufour gland contents of ants of the Cataglyphis bicolor group. J Chem Ecol 28:71–87
Guillot FS, Vinson SB (1972) The role of the calyx poison gland of Cardiochiles nigriceps in the host parasitoid relationship. J Insect Physiol 18:1315–1321
Hefetz A (1998) Exocrine glands and their products in non-Apis bees: chemical, functional and evolutionary perspectives. In: Vander Meer RK, Breed MD, Winston ML, Espelie KE (eds) Pheromone communication in social insects. Comstock, Ithaca, pp 236–256
Hefetz A, Lenoir A (1992) Dufour’s gland composition in the desert ant Cataglyphis: species specificity and population differences. Z Naturforsch 47C:285–289
Hefetz A, Fales HM, Batra SWT (1979) Natural polyesters: Dufour’s gland macrocyclic lactones form brood cell laminesters in Colletes bees. Science 204:415–417
Heinze J, Oberstadt B, Tentschert J, Hölldobler B, Bestmann HJ (1998) Colony specificity of Dufour gland secretions in a functionally monogynous ant. Chemoecology 8:169–174
Hölldobler B, Wilson EO (1990) The ants. Springer, Berlin Heidelberg London
Hubbard SF, Marris GC, Reynolds AJ, Rowe GW (1987) Adaptive patterns in the avoidance of superparasitism by solitary parasitic wasps. J Anim Ecol 56:387–404
Hustert R, Gnatzy W (1995) The motor program for defensive kicking in crickets: performance and neural control. J Exp Biol 198:1275–1283
Ikenga JO, Chapman G (1989) Ultrastructural aspects of Dufour’s gland of three day old worker honey bees, Apis mellifera ligustica (Hymenoptera: Apidae). J Kans Entomol Soc 62:449–461
Jackson BD, Billen JPJ, Morgan ED (1989) Dufour gland contents of three species of Myrmecia (Hymenoptera: Formicidae), primitive ants of Australia. J Chem Ecol 15:2191–2205
Jessen K, Maschwitz U (1983) Abdominaldrüsen bei Pachycondila tridentata (Formicidae: Ponerinae). Insectes Sociaux 30:123–133
Katzav-Gozansky T, Soroker V, Ibarra F, Franke W, Hefetz A (2001) Dufour’s gland secretion of the queen honey bee (Apis mellifera): an egg discriminator pheromone or a queen signal? Behav Ecol Sociobiol 51:76–86
Keegans SJ, Morgan ED, Turillazzi S, Jackson BD, Billen J (1993) The Dufour gland and the secretion placed on eggs of two species of social wasps Liostenogaster flavolineata and Parischnogaster jacobsoni Vespidae Stenogastrinae. J Chem Ecol 19:279–290
Koeniger G, Hänel H, Wissel M, Herth W (1996) Cornual gland of the honeybee drone (Apis mellifera L): structure and secretion. Apidologie 27:145–156
Koepsell H, Korn K, Ferguson D, Menuhr H, Ollig D, Haase W (1984) Reconstitution and partial purification of several Na+ co-transport systems from renal brush-border membranes. J Biol Chem 259:6548–6558
Lanne BS, Bergström G, Löfqvist J (1988) Dufour gland alkenes from the four ant species: F. polyctena, F. lugubris, F. truncorum and F. uralensis. Comp Biochem Physiol 91B:729–734
Lockey KH (1988) Lipids of the insect cuticle: origin, composition and function. Comp Biochem Physiol 89B:595–645
Maile R, Jungnickel H, Morgan ED, Ito F, Billen J (2000) Secretion of venom and Dufour glands in the ant Leptogenys diminuta. J Chem Ecol 26:2497–2506
Maschwitz U, Kloft W (1971) Morphology and function of the venom apparatus of insects, bees, wasps, ants and caterpillars. In: Bücherl W, Buckley EE (eds) Venomous animals and their venoms, vol 3. Academic Press, New York, pp 31–60
Menke AS, Bohart RM (1979) Sphecid wasps of the world: errors and omissions (Hymenoptera: Sphecidae). Proc Entomol Soc Wash 81:111–124
Mudd AR, Fisher C, Smith MC (1982) Volatile hydrocarbons in the Dufour’s gland of the parasite Nemeritis canescens (Grav.) (Hymenoptera: Ichneumonidae). J Chem Ecol 8:1035–1042
Nelson DR, Blomquist RJ (1995) Insect waxes. In: Hamilton RJ (ed) Waxes: chemistry, molecular biology and functions. The Oily Press, Dundee, pp 1–90
Nelson DR, Adams TS, Fatland CL (2003) Hydrocarbons in the surface wax of eggs and adults of the Colorado potato beetle, Leptinotarsa decemlineata. Comp Biochem Physiol B Biochem Mol Biol 134:447–466
Noirot C, Quennedey A (1974) Fine structure of insect epidermal glands. Annu Rev Entomol 19:61–80
Norden B, Batra SWT, Fales HM, Hefetz A, Shaw J (1980) Anthophora bee: unusual glycerides from maternal Dufour’s gland serve as larval food and cell lining. Science 207:1095–1097
Oldroyd BP, Ratnieks FL, Wossler TC (2002) Egg-marking pheromones in honey-bees Apis mellifera. Behav Ecol Sociobiol 51:590–591
Osman S, Führer E (1979) Histochemical analysis of accessory genital gland secretion in female Pimpla turionellae L. (Hymenoptera: Ichneumonidae). Int J Invert Reprod 1:323–332
Pampel W (1913) Die weiblichen Geschlechtsorgane der Ichneumonidae. Z Wiss Zool 108:290–357
Piek T (1986) Venoms of the Hymenoptera. Biochemical, pharmacological and behavioral aspects. Academic Press, London, pp 1–570
Rathmayer W (1962) Das Paralysierungsproblem beim Bienenwolf Philanthus triangulum F (Hym. Sphecidae). Z Vergl Physiol 45:413–462
Rathmayer W (1978) Venoms of Sphecidae, Pompilidae, Mutillidae and Bethylidae. In: Bettini S (ed) Arthropod venoms. Handbook of experimental pharmacology, vol 48. Springer, Berlin Heidelberg New York, pp 661–690
Robertson PL (1968) A morphological and functional study of the venom apparatus in some representative major groups of Hymenoptera. Aust J Zool 16:133–166
Roces F, Gnatzy W (1997) Reduced metabolic rate in crickets paralysed by a digger wasp. Naturwissenschaften 84:382–366
Schaffner W, Weissmann C (1973) A rapid, and specific method for the determination of protein in dilute solution. Anal Biochem 56:506–514
Schulz S (2001) Composition of the silk lipids of the spider Nephila clavipes. Lipids 36:637–647
Syvertsen TC, Jackson LL, Blomquist GJ, Vinson SB (1995) Alkadienes mediating courtship in the parasitoid Cardiochelis nigriceps (Hymenoptera, Braconidae). J Chem Ecol 21:1971–1989
Tagawa J (1983) Female sex pheromone glands in the parasitic wasps, genus Apanteles. Appl Entomol Zool 18:416–427
Tengö J, Hefetz A, Bertsch A, Schmitt U, Lübke G, Franke W (1991) Species specificity and complexity of Dufour’s gland secretion of bumble bees. Comp Biochem Physiol 99B:641–646
Trump BF, Smuckler EA, Benditt EP (1961) A method for staining epoxy sections for light microscopy. J Ultrastruct Res 5:343–348
Van Marle J, Piek T (1986) Morphology of the venom apparatus. In: Piek T (ed) Venoms of the Hymenoptera. Academic Press, London, pp 17–44
Vincenti M, Guglielmetti G, Cassani G, Tonini C (1987) Determination of double-bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal Chem 59:694–699
Vinson SB, Guillot FS (1972) Host-marking: source of a substance that results in host discrimination in insect parasitoids. Entomophaga 17:241–245
Vinson SB, Frankie GW, Blum MS, Wheeler JW (1978) Isolation, identification and function of the Dufour gland secretion of Xylocopa virginica texana (Hymenoptera: Anthophoridae). J Chem Ecol 4:315–323
Volknandt W, Henkel H, Zimmermann H (1988) Heterogeneous distribution of synaptophysin and protein 65 in synaptic vesicles isolated from rat cerebral cortex. Neurochem Int 12:337–345
Volknandt W, Schläfer M, Bonzelius F, Zimmermann H (1990) Svp25, a synaptic vesicle membrane glycoprotein from Torpedo electric organ that binds calcium and forms a homooligomeric complex. EMBO J 9:2465–2470
Weseloh RM (1976) Dufour’s gland: source of sex pheromone in a hymenopterous parasitoid. Science 193:695–697
Weseloh RM (1980) Sex pheromone gland and gypsy moth parasitoid, Apanteles melanoscelus: revaluation and ultrastructural survey. Ann Entomol Soc Am 73:576–580
Acknowledgements
We wish to thank Dr. U. Maschwitz for valuable comments and Dr. M. Jatho for numerous constructive suggestions on the styling of the figures especially the schematic drawing. We are grateful to O. Dittberner, B. Göckel, B. Krebs, U. Peters, M. Stör and A. Winter for skillful technical assistance. We also wish to thank M. Ruppel for assistance with the scanning microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gnatzy, W., Volknandt, W. & Schulz, S. Dufour gland of the digger wasp Liris niger: structure and developmental and biochemical aspects. Cell Tissue Res 315, 125–138 (2004). https://doi.org/10.1007/s00441-003-0813-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0813-2