Abstract
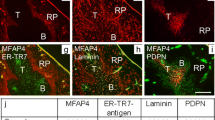
The extracellular matrix (ECM) of the spleen of the guinea fowl has been studied by immunohistochemistry. Each splenic compartment contains a different composition of ECM. Reticular-fiber-specific collagen III is expressed by the red pulp and thymus-dependent periarterial lymphatic sheath, but silver impregnation reveals a reticular-fiber-like structure in the periellipsoidal white pulp (PWP) where collagen III is absent. The penicillar capillaries of one central artery are enveloped by a single branching sheath or sleeve: the ellipsoid or Schweigger-Seidel sheath. The shape of the sleeve shows more resemblance to a deer antler than to an ellipsoid; it emerges at the beginning of the penicillar capillaries and ends at the edge of the PWP. It is wrapped by a novel discontinuous basement-membrane-like structure that expresses tenascin and that is named the capsule of the Schweigger-Seidel sheath (CSS). The cuboidal-shaped inner cell layer of the ellipsoid can be identified by a novel monoclonal antibody: BID3. BID3-positive stellate-shaped cells also occur in the PWP, suggesting that this cell population has migratory capability. Monoclonal antibody KIF8 recognizes an ECM component in the ellipsoid not only of guinea fowl, but also of chicken and quail, and may thus identify an ellipsoid-specific antigen. Collagen I is associated with both the basement membrane of the penicillar capillary and the CSS, whereas collagen III is present only in the CSS. Laminin is expressed in the red pulp, but its staining pattern does not indicate the presence of the "ring fibers", which suggests the absence of sinuses. Fibronectin is the only ECM molecule studied that occurs in every splenic compartment, indicating extensive intrasplenic cell migration.







Similar content being viewed by others
References
Billroth T (1857) Beiträge zur vergleichenden Histologie der Milz. Müllers Arch 88:88–108
Brelinska R, Pilgrim C (1982) The significance of the subcompartments of the marginal zone for directing lymphocyte traffic within the splenic pulp of the rat. Cell Tissue Res 226:155–165
Blue J, Weiss L (1981a) Periarterial macrophage sheaths (ellipsoids) in cat spleen—an electron microscope study. Am J Anat 161:115–134
Blue J, Weiss L (1981b) Electron microscopy of the red pulp of the dog spleen including vascular arrangements, periarterial macrophage sheaths (ellipsoids), and the contractile, innervated reticular meshwork. Am J Anat 161:189–218
Burthem J, Baker PK, Hunt JA, Cawley JC (1994) Hairy cell interactions with extracellular matrix: expression of specific integrin receptors and their role in the cell's response to specific adhesive proteins. Blood 84:873–882
Chilosi M, Lestani M, Benedetti A, Montagna L, Pedron S, Scarpa A, Menestrina F, Hirohashi S, Pizzolo G, Semenzato G (1993) Constitutive expression of tenascin in T-dependent zones of human lymphoid tissues. Am J Pathol 143:1348–1355
Claassen E, Kors N, Dijkstra CD, Van Rooijen N (1986) Marginal zone of the spleen and the development and localization of specific antibody-forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology 57:399–403
Clark RA, Erickson HP, Springer TA (1997) Tenascin supports lymphocyte rolling. J Cell Biol 137:755–765
Ewijk W van, Nieuwenhuis P (1985) Compartments, domains and migration pathways of lymphoid cells in the splenic pulp. Experientia 41:199–208
Gallego M, Cacho E del, Lopez-Bernad F, Bascuas JA (1997) Identification of avian dendritic cells in the spleen using a monoclonal antibody specific for chicken follicular dendritic cells. Anat Rec 249:81–95
Hemesath TJ, Stefansson K. (1994) Expression of tenascin in thymus and thymic nonlymphoid cells. J Immunol 152:422–428
Hemesath TJ, Marton LS, Stefansson K (1994) Inhibition of T cell activation by the extracellular matrix protein tenascin. J Immunol 152:5199–5207
Jeurissen, SHM, Janse EM, Koch G, deBoer GF (1988) The monoclonal antibody CVI-ChNL-68.1 recognizes cells of the monocyte-macrophage lineage in chickens. Dev Comp Immunol 12:855–864
Jeurissen SH, Claassen E, Janse EM (1992) Histological and functional differentiation of non-lymphoid cells in the chicken spleen. Immunology 77:75–80
Jeurissen, SHM, Vervelde L, Janse EM (1994) Structure and function of lymphoid tissues of the chicken. Poultry Sci Rev 5:183–207
Kiernan JA (1990) Histological and histochemical methods: theory and practice. Pergamon, Oxford New York Beijing Frankfurt Sao Paolo Sydney Toronto
Klein G, Beck S, Muller CA (1993) Tenascin is a cytoadhesive extracellular matrix component of the human hematopoietic microenvironment. J Cell Biol 123:1027–1035
Kumararatne DS, Bazin H, MacLennan IC (1981) Marginal zones: the major B cell compartment of rat spleens. Eur J Immunol 11:858–864
Liakka KA, Autio-Harmainen HI (1992) Distribution of the extracellular matrix proteins tenascin, fibronectin, and vitronectin in fetal, infant, and adult human spleens. J Histochem Cytochem 40:1203–1210
Liakka A, Karjalainen H, Virtanen I, Autio-Harmainen H (1995) Immuno-electron-microscopic localization of types III pN-collagen and IV collagen, laminin and tenascin in developing and adult human spleen. Cell Tissue Res 282:117–127
Nagy N, Magyar A, David C, Gumati MK, Oláh I (2001) Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of Fabricius of the guinea fowl (Numida meleagris) studied by novel monoclonal antibodies. Anat Rec 262:279–292
Nanbo K, Itagaki T, Ono T, Hara H, Iijima S (1979) Comparative morphology of the perifollicular region of spleens of birds and mammals. Recent Adv Res 19:15–42
Ocklind G, Talts J, Fassler R, Mattsson A, Ekblom P (1993) Expression of tenascin in developing and adult mouse lymphoid organs. J Histochem Cytochem 41:1163–1169
Oláh I, Glick B (1982) Splenic white pulp and associated vascular channels in chicken spleen. Am J Anat 165:445–480
Oláh I, Röhlich P, Törő I (1975) Fine structure of the lymphoid organs. An electron microscopic study. Lippincott, Philadelphia
Oláh I, Glick B, Taylor R Jr (1985) Effect of surgical bursectomy on the ellipsoid-associated cells, and peri-ellipsoid region of the chicken's spleen. J Leukoc Biol 38:459–469
Ruegg CR, Chiquet-Ehrismann R, Alkan SS (1989) Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci USA 19:7437–7441
Schweigger-Seidel F (1863) Untersuchungen über die Milz. II. Von den Arterienenden der Pulpa and den Bahnen des Blutes. Virchows Arch 27:460–504
Acknowledgements
The authors thank Ildikó Schay for typing the paper, and Jutka Ince, Margit Pál, and Beáta Urák for laboratory and photographic assistance. Monoclonal antibodies 31, B3/D6, and QCPN were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA 52242, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to this work
This work was supported partly by OTKA grant no. T-042558, and partly by ETT grant no. 145/99
Rights and permissions
About this article
Cite this article
Gumati, M.K., Magyar, A., Nagy, N. et al. Extracellular matrix of different composition supports the various splenic compartments of guinea fowl (Numida meleagris). Cell Tissue Res 312, 333–343 (2003). https://doi.org/10.1007/s00441-003-0736-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0736-y