Abstract
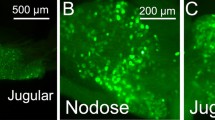
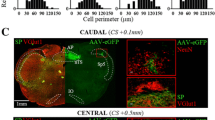
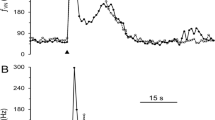
The vanilloid receptor VR1 is a nonselective cation channel activated by capsaicin as well as increases in temperature and acidity, and can be viewed as molecular integrator of chemical and physical stimuli that elicit pain. The distribution of VR1 receptors in peripheral and central processes of rat primary vagal afferent neurons innervating the gastrointestinal tract was investigated by immunohistochemistry. Forty-two percent of neurons in the nodose ganglia retrogradely labeled from the stomach wall expressed low to moderate VR1 immunoreactivity (VR1-IR). VR1-IR was considerably lower in the nodose ganglia as compared to the jugular and dorsal root ganglia. In the vagus nerve, strongly VR1-IR fibers ran in separate fascicles that supplied mainly cervical and thoracic targets, leaving only weakly VR1-IR fibers in the subdiaphragmatic portion. Vagal afferent intraganglionic laminar endings (IGLEs) in the gastric and duodenal myenteric plexus did not express VR1-IR. Similarly, VR1-IR was contained in fibers running in perfect register with vagal afferents, but was not colocalized with horseradish peroxidase in the same varicosities of intramuscular arrays (IMAs) and vagal afferent fibers in the duodenal submucosa anterogradely labeled from the nodose ganglia. Only in the gastric mucosa did we find evidence for colocalization of VR1-IR in vagal afferent terminals. In contrast, many nerve fibers coursing through the myenteric and submucosal plexuses contained detectable VR1-IR, the majority of which colocalized calcitonin gene-related peptide immunoreactivity. In the dorsal medulla there was a dense plexus of VR1-IR varicose fibers in the commissural, dorsomedial and gelatinosus subnuclei of the medial NTS and the lateral aspects of the area postrema, which was substantially reduced, but not eliminated on the ipsilateral side after supranodose vagotomy. It is concluded that about half of the vagal afferents innervating the gastrointestinal tract express low levels of VR1-IR, but that presence in most of the peripheral terminal structures is below the immunohistochemical detection threshold.






Similar content being viewed by others
References
Anavi-Goffer S, McKay NG, Ashford ML, Coutts AA (2002) Vanilloid receptor type 1-immunoreactivity is expressed by intrinsic afferent neurones in the guinea-pig myenteric plexus. Neurosci Lett 319:53–57
Anton PM, Theodorou V, Fioramonti J, Bueno L (2001) Chronic low-level administration of diquat increases the nociceptive response to gastric distension in rats: role of mast cells and tachykinin receptor activation. Pain 92:219–227
Berthoud HR, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319:261–276
Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17
Berthoud HR, Kressel M, Raybould HE, Neuhuber WL (1995) Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 191:203–212
Berthoud HR, Patterson LM, Neumann F, Neuhuber WL (1997) Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 195:183–191
Berthoud HR, Lynn PA, Blackshaw LA (2001) Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol Regul Integr Comp Physiol 280:R1371–R1381
Bielefeldt K (2000) Differential effects of capsaicin on rat visceral sensory neurons. Neuroscience 101:727–736
Blackshaw LA, Grundy D (1990) Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst 31:191–201
Blackshaw LA, Page AJ, Partosoedarso ER (2000) Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience 96:407–416
Calatayud S, Barrachina MD, Garcia-Zaragoza E, Quintana E, Esplugues JV (2001) Endotoxin inhibits gastric emptying in rats via a capsaicin-sensitive afferent pathway. Naunyn Schmiedebergs Arch Pharmacol 363:276–280
Carlton SM, Coggeshall RE (2001) Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett 310:53–56
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313.
Chen HF, Lee BP, Kou YR (1999) Two subgroups of lung vagal C-fibers with different vulnerabilities to blockades by perivagal capsaicin and vagal cooling in dogs. Chin J Physiol 42:219–225
Fox AJ, Barnes PJ, Urban L, Dray A (1993) An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol 469:21–35
Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P (1998) Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett 250:177–180
Ho CY, Gu Q, Lin YS, Lee LY (2001) Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127:113–124
Holzer P (1998) Neural injury, repair, and adaptation in the GI tract. II. The elusive action of capsaicin on the vagus nerve. Am J Physiol 275:G8–G13
Kaczynska K, Szereda-Przestaszewska M (2000) Respiratory effects of capsaicin occur beyond the lung vagi in anaesthetized rats. Acta Neurobiol Exp (Warsz) 60:159–165
Kressel M (1998) Tyramide amplification allows anterograde tracing by horseradish peroxidase-conjugated lectins in conjunction with simultaneous immunohistochemistry. J Histochem Cytochem 46:527–534
Kulkarni-Narla A, Brown DR (2001) Opioid, cannabinoid and vanilloid receptor localization on porcine cultured myenteric neurons. Neurosci Lett 308:153–156
Lemann M, Dederding JP, Flourie B, Franchisseur C, Rambaud JC, Jian R (1991) Abnormal perception of visceral pain in response to gastric distension in chronic idiopathic dyspepsia. The irritable stomach syndrome. Dig Dis Sci 36:1249–1254
Li Y, Owyang C (1996) Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin- dependent duodenal stimuli via vagal afferent fibres in the rat. J Physiol 494:773–782
Liu YX, Owyang C (1999) Duodenal acid-induced gastric relaxation is mediated by multiple pathways. Am J Physiol 276:G1501–G1506
Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D (1987) The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience 23:275–289
Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1- like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 97:3655–3660
Michael GJ, Priestley JV (1999) Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 19:1844–1854
Michl T, Jocic M, Heinemann A, Schuligoi R, Holzer P (2001) Vagal afferent signaling of a gastric mucosal acid insult to medullary, pontine, thalamic, hypothalamic and limbic, but not cortical, nuclei of the rat brain. Pain 92:19–27
Nozawa Y, Nishihara K, Yamamoto A, Nakano M, Ajioka H, Matsuura N (2001) Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci Lett 309:33–36
Ozaki N, Gebhart GF (2001) Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol 281:G1449–G1459
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic, San Diego
Phillips RJ, Powley TL (2000) Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev 34:1–26
Powley TL, Berthoud HR (1991) A fluorescent labeling strategy for staining the enteric nervous system. J Neurosci Methods 36:9–15
Raybould HE, Tache Y (1989) Capsaicin-sensitive vagal afferent fibers and stimulation of gastric acid secretion in anesthetized rats. Eur J Pharmacol 167:237–243
Raybould HE, Holzer HH (1993) Duodenal acid-induced inhibition of gastric motility and emptying in rats. Am J Physiol 265:G540–G546
Sekizawa S, Ishikawa T, Sant'Ambrogio FB, Sant'Ambrogio G (1999) Vagal esophageal receptors in anesthetized dogs: mechanical and chemical responsiveness. J Appl Physiol 86:1231–1235
Sharkey KA, Sobrino JA, Cervero F, Varro A, Dockray GJ (1989) Visceral and somatic afferent origin of calcitonin gene-related peptide immunoreactivity in the lower thoracic spinal cord of the rat. Neuroscience 32:169–179
Sharkey KA, Oland LD, Kirk DR, Davison JS (1991) Capsaicin-sensitive vagal stimulation-induced gastric acid secretion in the rat: evidence for cholinergic vagal afferents. Br J Pharmacol 103:1997–2003
South EH, Ritter RC (1988) Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9:601–612
Such G, Jancso G (1986) Axonal effects of capsaicin: an electrophysiological study. Acta Physiol Hung 67:53–63
Szallasi A, Blumberg PM (1996) Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain 68:195–208
Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hökfelt T, Lundberg JM (1995) Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res 703:175–183
Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y (2001) Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem 76:1628–1635
Tominaga M, Julius D (2000) Capsaicin receptor in the pain pathway. Jpn J Pharmacol 83:20–24
Traub RJ, Sengupta JN, Gebhart GF (1996) Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience 74:873–884
Waddell PJ, Lawson SN (1989) The C-fibre conduction block caused by capsaicin on rat vagus nerve in vitro. Pain 39:237–242
Zagorodnyuk VP, Chen BN, Brookes SJ (2001) Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 534:255–268
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was partially funded by the National Institute for Diabetes and Digestive and Kidney Diseases, DK47348.
Rights and permissions
About this article
Cite this article
Patterson, L.M., Zheng, H., Ward, S.M. et al. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res 311, 277–287 (2003). https://doi.org/10.1007/s00441-002-0682-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-002-0682-0