Abstract
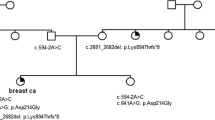
Germline gain of function variants in the oncogene ABL1 cause congenital heart defects and skeletal malformations (CHDSKM) syndrome. Whether a corresponding ABL1 deficiency disorder exists in humans remains unknown although developmental defects in mice deficient for Abl1 support this notion. Here, we describe two multiplex consanguineous families, each segregating a different homozygous likely loss of function variant in ABL1. The associated phenotype is multiple congenital malformations and distinctive facial dysmorphism that are opposite in many ways to CHDSKM. We suggest that a tight balance of ABL1 activity is required during embryonic development and that both germline gain of function and loss of function variants result in distinctively different allelic congenital malformation disorders.



Similar content being viewed by others
Data availability
All data supporting the findings described in this manuscript are available in the article and from the corresponding author upon request.
References
AlAbdi L, Maddirevula S, Shamseldin HE, Khouj E, Helaby R, Hamid H, Almulhim A, Hashem MO, Abdulwahab F, Abouyousef O (2023) Diagnostic implications of pitfalls in causal variant identification based on 4577 molecularly characterized families. Nat Commun 14:5269
Apperley J (2009) Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw 7:1050–1058
Brasher BB, Van Etten RA (2000) c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem 275:35631–35637
Chen CA, Crutcher E, Gill H, Nelson TN, Robak LA, Jongmans MC, Pfundt R, Prasad C, Berard RA, Fannemel M (2020) The expanding clinical phenotype of germline ABL1-associated congenital heart defects and skeletal malformations syndrome. Hum Mutat 41:1738–1744
Dingemans AJM, Hinne M, Truijen KMG, Goltstein L, van Reeuwijk J, de Leeuw N, Schuurs-Hoeijmakers J, Pfundt R, Diets IJ, den Hoed J, de Boer E, Coenen-van der Spek J, Jansen S, van Bon BW, Jonis N, Ockeloen CW, Vulto-van Silfhout AT, Kleefstra T, Koolen DA, Campeau PM, Palmer EE, Van Esch H, Lyon GJ, Alkuraya FS, Rauch A, Marom R, Baralle D, van der Sluijs PJ, Santen GWE, Kooy RF, van Gerven MAJ, Vissers L, de Vries BBA (2023) PhenoScore quantifies phenotypic variation for rare genetic diseases by combining facial analysis with other clinical features using a machine-learning framework. Nat Genet 55:1598–1607. https://doi.org/10.1038/s41588-023-01469-w
El Gendy M, Kandil A, Helal M, Zahou F (2015) The teratogenic effects of imatinib mesylate on rat fetuses. Toxicol Rep 2:654–663
Greuber EK, Smith-Pearson P, Wang J, Pendergast AM (2013) Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 13:559–571
Hildebrandt CC, Patel N, Graham JM Jr, Bamshad M, Nickerson DA, White JJ, Marvin CT, Miller DE, UoWCfM G, Grand KL (2021) Further delineation of van den Ende-Gupta syndrome: Genetic heterogeneity and overlap with congenital heart defects and skeletal malformations syndrome. Am J Med Genet A 185:2136–2149
Klein AD, Kessel AGV, Grosveld G, Bartram CR, Hagemeijer A, Bootsma D, Spurr NK, Heisterkamp N, Groffen J, Stephenson JR (1982) A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature 300:765–767
Li B, Boast S, De LosSantos K, Schieren I, Quiroz M, Teitelbaum SL, Tondravi MM, Goff SP (2000) Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Gene 24:304–308
Melo Rito MT, Freitas I, Pinto-Basto J, Kay T, Antunes D (2019) Germline ABL1variant identified in a Nepalese girl with congenital heart defects and skeletal malformations syndrome: a case report 23rd Annual Meeting of the Portuguese Society of Human Genetics, Coimbra, Portugal. Wolters Kluwer Health, Inc., pp p e19291
Nussinov R, Tsai C-J, Jang H (2022) How can same-gene mutations promote both cancer and developmental disorders? Sci Adv. https://doi.org/10.1126/sciadv.abm2059
Pye SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, Rosti G, Apperley JF (2008) The effects of imatinib on pregnancy outcome. Blood J Am Soc Hematol 111:5505–5508
Qi H, Dong C, Chung WK, Wang K, Shen Y (2016) Deep genetic connection between cancer and developmental disorders. Hum Mutat 37:1042–1050
Qiu Z, Cang Y, Goff SP (2010) c-Abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci 107:1136–1141
Schwartzberg PL, Goff SP, Robertson EJ (1989) Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246:799–803
Schwartzberg PL, Stall AM, Hardin JD, Bowdish KS, Humaran T, Boast S, Harbison ML, Robertson EJ, Goff SP (1991) Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65:1165–1175
Spencer C, Comitis G, Lawrenson J, Fieggen K (2023) ABL1-related congenital heart defects and skeletal malformations syndrome in a patient from Sub-Saharan Africa: a case report highlighting novel cardiac features. Am J Med Genet A 191:1652–1655
Strande NT, Riggs ER, Buchanan AH, Ceyhan-Birsoy O, DiStefano M, Dwight SS, Goldstein J, Ghosh R, Seifert BA, Sneddon TP (2017) Evaluating the clinical validity of gene-disease associations: an evidence-based framework developed by the clinical genome resource. Am J Hum Genet 100:895–906
Testoni E, Stephenson NL, Torres-Ayuso P, Marusiak AA, Trotter EW, Hudson A, Hodgkinson CL, Morrow CJ, Dive C, Brognard J (2016) Somatically mutated ABL 1 is an actionable and essential NSCLC survival gene. EMBO Mol Med 8:105–116
Thaxton C, Goldstein J, DiStefano M, Wallace K, Witmer PD, Haendel MA, Hamosh A, Rehm HL, Berg JS (2022) Lumping versus splitting: how to approach defining a disease to enable accurate genomic curation. Cell Genomics 2:100131
Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC (1991) Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65:1153–1163
Wang X, Charng W-L, Chen C-A, Rosenfeld JA, Al Shamsi A, Al-Gazali L, McGuire M, Mew NA, Arnold GL, Qu C (2017) Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations. Nat Genet 49:613–617
Webb MJ, Jafta D (2012) Imatinib use in pregnancy. Turk J Haematol 29:405
Acknowledgements
We would like to thank the families included in this study for their enthusatic assistance. The authors also thank KFMC Research Centre for partial support (IRF 019-052) and Alexander J. M. Dingemans for his help with the analysis performed using PhenoScore.
Funding
This work was funded by King Fahad Medical City, Under grant No. IRF 019-052.
Author information
Authors and Affiliations
Contributions
LA, TN, EF, and FSA wrote the main manuscript text and FA, ECP, and US prepared figures 1-3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
AlAbdi, L., Neuhann, T., Prott, EC. et al. Human ABL1 deficiency syndrome (HADS) is a recognizable syndrome distinct from ABL1-related congenital heart defects and skeletal malformations syndrome. Hum. Genet. (2024). https://doi.org/10.1007/s00439-024-02677-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00439-024-02677-y