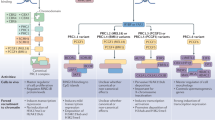
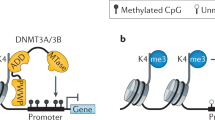
Abstract
Metazoan development arises from spatiotemporal control of gene expression, which depends on epigenetic regulators like the polycomb group proteins (PcG) that govern the chromatin landscape. PcG proteins facilitate the addition and removal of histone 2A monoubiquitination at lysine 119 (H2AK119ub1), which regulates gene expression, cell fate decisions, cell cycle progression, and DNA damage repair. Regulation of these processes by PcG proteins is necessary for proper development, as pathogenic variants in these genes are increasingly recognized to underly developmental disorders. Overlapping features of developmental syndromes associated with pathogenic variants in specific PcG genes suggest disruption of central developmental mechanisms; however, unique clinical features observed in each syndrome suggest additional non-redundant functions for each PcG gene. In this review, we describe the clinical manifestations of pathogenic PcG gene variants, review what is known about the molecular functions of these gene products during development, and interpret the clinical data to summarize the current evidence toward an understanding of the genetic and molecular mechanism.

Similar content being viewed by others
Data availability
No novel data to share.
References
Abdel-Wahab O, Gao J, Adli M et al (2013) Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo Conditional deletion of Asxl1 results in MDS. J Exp Med 210(12):2641–2659
Akasaka T, Kanno M, Balling R, Mieza MA, Taniguchi M, Koseki H (1996) A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122(5):1513–1522
Arlotta P, Hobert O (2015) Homeotic transformations of neuronal cell identities. Trends Neurosci 38(12):751–762
Awad S, Al-Dosari MS, Al-Yacoub N et al (2013) Mutation in PHC1 implicates chromatin remodeling in primary microcephaly pathogenesis. Hum Mol Genet 22(11):2200–2213
Baskind HA, Na L, Ma Q, Patel MP, Geenen DL, Wang QT (2009) Functional conservation of Asxl2, a murine homolog for the Drosophila enhancer of trithorax and polycomb group gene Asx. PLoS ONE 4(3):e4750
Baymaz HI, Fournier A, Laget S et al (2014) MBD5 and MBD6 interact with the human PR-DUB complex through their methyl-CpG-binding domain. Proteomics 14(19):2179–2189
Bedogni F, Hodge RD, Elsen GE et al (2010) Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci 107(29):13129–13134
Beunders G, Voorhoeve E, Golzio C et al (2013) Exonic deletions in AUTS2 cause a syndromic form of intellectual disability and suggest a critical role for the C terminus. Am J Hum Genet 92(2):210–220
Beunders G, De Munnik SA, Van der Aa N et al (2015) Two male adults with pathogenic AUTS2 variants, including a two-base pair deletion, further delineate the AUTS2 syndrome. Eur J Hum Genet 23(6):803–807
Beunders G, Van De Kamp J, Vasudevan P et al (2016) A detailed clinical analysis of 13 patients with AUTS2 syndrome further delineates the phenotypic spectrum and underscores the behavioural phenotype. J Med Genet 53(8):523–532
Biel A, Castanza AS, Rutherford R et al (2022) AUTS2 syndrome: molecular mechanisms and model systems. Published online, Front Mol Neurosci
Blackledge NP, Farcas AM, Kondo T et al (2014) Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157(6):1445–1459
Bölicke N, Albert M (2022) Polycomb-mediated gene regulation in human brain development and neurodevelopmental disorders. Dev Neurobiol 82(4):345–363
Bravo M, Nicolini F, Starowicz K et al (2015) Polycomb RING1A-and RING1B-dependent histone H2A monoubiquitylation at pericentromeric regions promotes S-phase progression. J Cell Sci 128(19):3660–3671
Buchwald G, van der Stoop P, Weichenrieder O, Perrakis A, van Lohuizen M, Sixma TK (2006) Structure and E3-ligase activity of the Ring-Ring complex of Polycomb proteins Bmi1 and Ring1b. EMBO J 25(11):2465–2474
Campagne A, Lee MK, Zielinski D et al (2019) BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun 10(1):1–15
Carlston CM, O’Donnell-Luria AH, Underhill HR et al (2017) Pathogenic ASXL1 somatic variants in reference databases complicate germline variant interpretation for Bohring-Opitz Syndrome. Hum Mutat 38(5):517–523
Dauber KL, Perdigoto CN, Valdes VJ, Santoriello FJ, Cohen I, Ezhkova E (2016) Dissecting the roles of polycomb repressive complex 2 subunits in the control of skin development. J Invest Dermatol 136(8):1647–1655
del Mar Lorente M, Marcos-Gutiérrez C, Pére C, et al (2000) Loss-and gain-of-function mutations show a polycomb group function for Ring1A in mice. Development 127(23):5093–5100
Di Lullo E, Kriegstein AR (2017) The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18(10):573–584
Doyle EJ, Morey L, Conway E (2022a) Know when to fold em: polycomb complexes in oncogenic 3D genome regulation. Front Cell Dev Biol. 10:1756
Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, Kirkpatrick DS, Pham VC, Lill JR, Bakalarski CE, Jiansheng W, Phu L, Katavolos P, LaFave LM, Abdel-Wahab O, Modrusan Z, Seshagiri S, Dong K, Lin Z, Balazs M, Suriben R, Newton K, Hymowitz S, Garcia-Manero G, Martin Fl, Levine RL, Dixit VM (2012) Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337(6101):1541–1546. https://doi.org/10.1126/science.1221711
Doyle LA, Unlu Bektas F, Chatzantonaki E, Repton C, Derrien A, Illingworth RS (2022b) RINGs, DUBs and Abnormal Brain Growth—Histone H2A Ubiquitination in Brain Development and Disease. Epigenomes 6(4):42
Efthymiou S, Salpietro V, Pironti E et al (2019) A de novo truncating mutation in ASXL1 associated with segmental overgrowth. J Genet 98(5):1–5
Ercoskun P, Yuce Kahraman C, Adanur Saglam K, Kanjee M, Tatar A (2022) A new case of Turnpenny-Fry syndrome. Am J Med Genet A 188(2):688–691
Eskeland R, Leeb M, Grimes GR et al (2010) Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell 38(3):452–464
Fahrner JA, Bjornsson HT (2019) Mendelian disorders of the epigenetic machinery: postnatal malleability and therapeutic prospects. Hum Mol Genet 28(R2):R254–R264
Fair SR, Schwind W, Julian DL et al (2023) Cerebral organoids containing an AUTS2 missense variant model microcephaly. Brain 146(1):387–404
Farcas AM, Blackledge NP, Sudbery I et al (2012) KDM2B links the Polycomb Repressive Complex 1 (PRC1) to recognition of CpG islands. Elife 1:00205
Francis NJ, Kingston RE, Woodcock CL (2004) Chromatin compaction by a polycomb group protein complex. Science 306(5701):1574–1577
Fursova NA, Blackledge NP, Nakayama M et al (2019) Synergy between variant PRC1 complexes defines polycomb-mediated gene repression. Mol Cell 74(5):1020–1036
Gafner M, Michelson M, Argilli E et al (2022) Major brain malformations: corpus callosum dysgenesis, agenesis of septum pellucidum and polymicrogyria in patients with BCORL1-related disorders. J Hum Genet 67(2):95–101
Ganna A, Genovese G, Howrigan DP et al (2016) Ultra-rare disruptive and damaging mutations influence educational attainment in the general population. Nat Neurosci 19(12):1563–1565
Gao Z, Zhang J, Bonasio R et al (2012) PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol Cell 45(3):344–356
Gao Z, Lee P, Stafford JM, Von Schimmelmann M, Schaefer A, Reinberg D (2014) An AUTS2–Polycomb complex activates gene expression in the CNS. Nature 516(7531):349–354
Gilmore EC, Walsh CA (2013) Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol 2(4):461–478
Ginjala V, Nacerddine K, Kulkarni A et al (2011) BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol Cell Biol 31(10):1972–1982
Goldstein AM (2011) Germline BAP1 mutations and tumor susceptibility. Nat Genet 43(10):925–926
Herculano-Houzel S, Mota B, Lent R (2006) Cellular scaling rules for rodent brains. Proc Natl Acad Sci 103(32):12138–12143
Hoischen A, van Bon BW, Rodríguez-Santiago B et al (2011) De novo nonsense mutations in ASXL1 cause Bohring-Opitz syndrome. Nat Genet 43(8):729–731
Hori K, Nagai T, Shan W et al (2014) Cytoskeletal regulation by AUTS2 in neuronal migration and neuritogenesis. Cell Rep 9(6):2166–2179
Hori K, Shimaoka K, Hoshino M (2021) AUTS2 gene: keys to understanding the pathogenesis of neurodevelopmental disorders. Cells 11(1):11
Illingworth RS, Moffat M, Mann AR et al (2015) The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev 29(18):1897–1902
Inoue D, Matsumoto M, Nagase R et al (2016) Truncation mutants of ASXL1 observed in myeloid malignancies are expressed at detectable protein levels. Exp Hematol 44(3):172–176
Ishida A, Asano H, Hasegawa M et al (1993) Cloning and chromosome mapping of the human Mel-18 gene which encodes a DNA-binding protein with a new ‘RING-finger’ motif. Gene 129(2):249–255
Ismail IH, Andrin C, McDonald D, Hendzel MJ (2010) BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J Cell Biol 191(1):45–60
Ismail IH, McDonald D, Strickfaden H, Xu Z, Hendzel MJ (2013) A small molecule inhibitor of polycomb repressive complex 1 inhibits ubiquitin signaling at DNA double-strand breaks. J Biol Chem 288(37):26944–26954
Isono K, Endo TA, Ku M et al (2013) SAM domain polymerization links subnuclear clustering of PRC1 to gene silencing. Dev Cell 26(6):565–577
Jensen DE, Rauscher FJ III (1999) Defining biochemical functions for the BRCA1 tumor suppressor protein: analysis of the BRCA1 binding protein BAP1. Cancer Lett 143:S13–S17
Jiao Z, Zhao X, Wang Y et al (2022) A de novo and novel nonsense variants in ASXL2 gene is associated with Shashi-Pena syndrome. Eur J Med Genet 65(4):104454
Junco SE, Wang R, Gaipa JC et al (2013) Structure of the polycomb group protein PCGF1 in complex with BCOR reveals basis for binding selectivity of PCGF homologs. Structure 21(4):665–671
Kadariya Y, Cheung M, Xu J et al (2016) Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res 76(9):2836–2844
Kakarougkas A, Ismail A, Chambers AL et al (2014) Requirement for PBAF in transcriptional repression and repair at DNA breaks in actively transcribed regions of chromatin. Mol Cell 55(5):723–732
Karczewski KJ, Francioli LC, Tiao G et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581(7809):434–443
Kobrinski DA, Yang H, Kittaneh M (2020) BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res 9(Suppl 1):S60
Kolovos P, Nishimura K, Sankar A et al (2020) PR-DUB maintains the expression of critical genes through FOXK1/2-and ASXL1/2/3-dependent recruitment to chromatin and H2AK119ub1 deubiquitination. Genome Res 30(8):1119–1130
Kundu S, Ji F, Sunwoo H et al (2017) Polycomb repressive complex 1 generates discrete compacted domains that change during differentiation. Mol Cell 65(3):432–446
Küry S, Ebstein F, Mollé A et al (2022) Rare germline heterozygous missense variants in BRCA1-associated protein 1, BAP1, cause a syndromic neurodevelopmental disorder. Am J Hum Genet 109(2):361–372
Lai HL, Grachoff M, McGinley AL et al (2012) Maintenance of adult cardiac function requires the chromatin factor Asxl2. J Mol Cell Cardiol 53(5):734–741
Lee SC, Toth Z (2022) PRC1-independent binding and activity of RYBP on the KSHV genome during de novo infection. PLoS Pathog 18(8):e1010801
Lee HG, Kahn TG, Simcox A, Schwartz YB, Pirrotta V (2015) Genome-wide activities of Polycomb complexes control pervasive transcription. Genome Res 25(8):1170–1181
Leon E, Diaz J, Castilla-Vallmanya L, Grinberg D, Balcells S, Urreizti R (2020) Extending the phenotypic spectrum of Bohring-Opitz syndrome: mild case confirmed by functional studies. Am J Med Genet A 182(1):201–204
Lewis BP, Green RE, Brenner SE (2003) Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci 100(1):189–192
Li M, Collins R, Jiao Y et al (2011) Somatic mutations in the transcriptional corepressor gene BCORL1 in adult acute myelogenous leukemia. Blood J Am Soc Hematol 118(22):5914–5917
Li H, Liefke R, Jiang J et al (2017) Polycomb-like proteins link the PRC2 complex to CpG islands. Nature 549(7671):287–291
Liu S, Aldinger KA, Cheng CV et al (2021) NRF1 association with AUTS2-Polycomb mediates specific gene activation in the brain. Mol Cell 81(22):4663–4676
Luo X, Schoch K, Jangam SV et al (2021) Rare deleterious de novo missense variants in RNF2/RING2 are associated with a neurodevelopmental disorder with unique clinical features. Hum Mol Genet 30(14):1283–1292
Machour FE, Ayoub N (2020) Transcriptional regulation at DSBs: mechanisms and consequences. Trends Genet 36(12):981–997
Matheus F, Rusha E, Rehimi R et al (2019) Pathological ASXL1 mutations and protein variants impair neural crest development. Stem Cell Rep 12(5):861–868
McGinty RK, Henrici RC, Tan S (2014) Crystal structure of the PRC1 ubiquitylation module bound to the nucleosome. Nature 514(7524):591–596
McGrath BT, Wu P, Salvi S et al (1826) ASXL3 controls cortical neuron fate specification through extrinsic self-renewal pathways. BioRxiv. 2021:127
Micol JB, Duployez N, Boissel N et al (2014) Frequent ASXL2 mutations in acute myeloid leukemia patients with t (8; 21)/RUNX1-RUNX1T1 chromosomal translocations. Blood J Am Soc Hematol 124(9):1445–1449
Monderer-Rothkoff G, Tal N, Risman M et al (2021) AUTS2 isoforms control neuronal differentiation. Mol Psychiatry 26(2):666–681
Morimoto-Suzki N, Hirabayashi Y, Tyssowski K et al (2014) The polycomb component Ring1B regulates the timed termination of subcerebral projection neuron production during mouse neocortical development. Development 141(22):4343–4353
Muthusamy B, Bellad A, Girimaji SC, Pandey A (2021) Shukla-Vernon syndrome: a second family with a novel variant in the BCORL1 gene. Genes 12(3):452
Notwell JH, Heavner WE, Darbandi SF et al (2016) TBR1 regulates autism risk genes in the developing neocortex. Genome Res 26(8):1013–1022
Ohta H, Sawada A, Kim JY et al (2002) Polycomb group gene rae28 is required for sustaining activity of hematopoietic stem cells. J Exp Med 195(6):759–770
Oksenberg N, Stevison L, Wall JD, Ahituv N (2013) Function and regulation of AUTS2, a gene implicated in autism and human evolution. PLoS Genet 9(1):e1003221
Pagan JK, Arnold J, Hanchard KJ et al (2007) A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem 282(20):15248–15257
Pengelly AR, Kalb R, Finkl K, Müller J (2015) Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes Dev 29(14):1487–1492
Pereira JD, Sansom SN, Smith J, Dobenecker MW, Tarakhovsky A, Livesey FJ (2010) Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc Natl Acad Sci 107(36):15957–15962
Perez-Garcia V, Fineberg E, Wilson R et al (2018) Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 555(7697):463–468
Perez-Garcia V, Lea G, Lopez-Jimenez P et al (2021) BAP1/ASXL complex modulation regulates epithelial-mesenchymal transition during trophoblast differentiation and invasion. Elife 10:e63254
Pierce SB, Stewart MD, Gulsuner S et al (2018) De novo mutation in RING1 with epigenetic effects on neurodevelopment. Proc Natl Acad Sci 115(7):1558–1563
Piunti A, Shilatifard A (2021) The roles of Polycomb repressive complexes in mammalian development and cancer. Nat Rev Mol Cell Biol 22(5):326–345
Rockel A, Hiorns RW, Powell T (1980) The basic uniformity in structure of the neocortex. Brain J Neurol 103(2):221–244
Rose NR, King HW, Blackledge NP et al (2016) RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. Elife 5:e18591
Russell B, Johnston JJ, Biesecker LG et al (2015) Clinical management of patients with ASXL1 mutations and Bohring-Opitz syndrome, emphasizing the need for Wilms tumor surveillance. Am J Med Genet A 167(9):2122–2131
Russell B, Graham JM (2013) Expanding our knowledge of conditions associated with the ASXL gene family. Genome Med 5:16. https://doi.org/10.1186/gm420
Sanchez-Jimeno C, Blanco-Kelly F, López-Grondona F et al (2021) Attention deficit hyperactivity and autism spectrum disorders as the core symptoms of AUTS2 syndrome: Description of five new patients and update of the frequency of manifestations and genotype-phenotype correlation. Genes 12(9):1360
Schaefer EJ, Wang HC, Karp HQ et al (2022) BCOR and BCORL1 mutations drive epigenetic reprogramming and oncogenic signaling by unlinking PRC1 1 from target genes. Blood Cancer Discov. 3(2):116–135
Scheuermann JC, de Ayala Alonso AG, Oktaba K et al (2010) Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 465(7295):243–247
Schirwani S, Albaba S, Carere DA et al (2021) Expanding the phenotype of ASXL3-related syndrome: a comprehensive description of 45 unpublished individuals with inherited and de novo pathogenic variants in ASXL3. Am J Med Genet A 185(11):3446–3458
Shashi V, Pena LD, Kim K et al (2016) De novo truncating variants in ASXL2 are associated with a unique and recognizable clinical phenotype. Am J Hum Genet 99(4):991–999
Shukla V, Rao M, Zhang H, Beers J, Wangsa D, Wangsa D, Buishand FO, Wang Y, Zhiya Y, Stevenson HS, Reardon ES, McLoughlin KC, Kaufman AS, Payabyab EC, Hong JA, Zhang M, Davis S, Edelman D, Chen G, Miettinen MM, Restifo NP, Ried T, Meltzer PA, Schrump DS (2017) ASXL3 is a novel pluripotency factor in human respiratory epithelial cells and a potential therapeutic target in small cell lung cancer abstract. Cancer Research 77(22):6267–6281. https://doi.org/10.1158/0008-5472.CAN-17-0570
Shukla A, Girisha KM, Somashekar PH, Nampoothiri S, McClellan R, Vernon HJ (2019) Variants in the transcriptional corepressor BCORL1 are associated with an X-linked disorder of intellectual disability, dysmorphic features, and behavioral abnormalities. Am J Med Genet A 179(5):870–874
Sportoletti P, Sorcini D, Falini B (2021) BCOR gene alterations in hematologic diseases. Blood 138(24):2455–2468
Srivastava A, Ritesh K, Tsan YC et al (2016) De novo dominant ASXL3 mutations alter H2A deubiquitination and transcription in Bainbridge-Ropers syndrome. Hum Mol Genet 25(3):597–608
Srivastava A, Brian M, Stephanie LB (2017) Histone H2A monoubiquitination in neurodevelopmental Disorders. Trends Genet 33(8):566–578. https://doi.org/10.1016/j.tig.2017.06.002
Stock JK, Giadrossi S, Casanova M et al (2007) Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol 9(12):1428–1435
Sultana R, Chang-En Y, Jun Y, Munson J, Chen D, Hua W, Estes A, Cortes F, de la Barra F, Dongmei Y, Haider ST, Trask BJ, Green ED, Raskind WH, Disteche CM, Wijsman E, Dawson G, Storm DR, Schellenberg GD, Villacres EC (2002) Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics 80(2):129–134. https://doi.org/10.1006/geno.2002.6810
Szczepanski AP, Wang L (2021) Emerging multifaceted roles of BAP1 complexes in biological processes. Cell Death Discov 7(1):1–10
Szczepanski AP, Zhao Z, Sosnowski T, Goo YA, Bartom ET, Wang L (2020) ASXL3 bridges BRD4 to BAP1 complex and governs enhancer activity in small cell lung cancer. Genome Med 12(1):1–20
Takihara Y, Tomotsune D, Shirai M et al (1997) Targeted disruption of the mouse homologue of the Drosophila polyhomeotic gene leads to altered anteroposterior patterning and neural crest defects. Development 124(19):3673–3682
Tamburri S, Lavarone E, Fernández-Pérez D et al (2020) Histone H2AK119 mono-ubiquitination is essential for polycomb-mediated transcriptional repression. Mol Cell 77(4):840–856
Tatton-Brown K, Loveday C, Yost S et al (2017) Mutations in epigenetic regulation genes are a major cause of overgrowth with intellectual disability. Am J Hum Genet 100(5):725–736
Tavares L, Dimitrova E, Oxley D et al (2012) RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell 148(4):664–678
Testa JR, Cheung M, Pei J et al (2011) Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 43(10):1022–1025
Tsuboi M, Kishi Y, Yokozeki W, Koseki H, Hirabayashi Y, Gotoh Y (2018) Ubiquitination-independent repression of PRC1 targets during neuronal fate restriction in the developing mouse neocortex. Dev Cell 47(6):758–772
Tsuboyama N, Wang R, Szczepanski AP et al (2022) Therapeutic targeting of BAP1/ASXL3 sub-complex in ASCL1-dependent small cell lung cancer. Oncogene 41(15):2152–2162
Turnpenny PD, Wright MJ, Sloman M et al (2018) Missense mutations of the Pro65 residue of PCGF2 cause a recognizable syndrome associated with craniofacial, neurological, cardiovascular, and skeletal features. Am J Hum Genet 103(5):786–793
Uhlén M, Fagerberg L, Hallström BM et al (2015) Proteomics Tissue-based map of the human proteome. Sci 347:1260419–1260419
Voncken JW, Roelen BA, Roefs M et al (2003) Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci 100(5):2468–2473
Wang Q, Geng Z, Gong Y et al (2018) WDR68 is essential for the transcriptional activation of the PRC1-AUTS2 complex and neuronal differentiation of mouse embryonic stem cells. Stem Cell Res 33:206–214
Wang L, Birch NW, Zhao Z et al (2021) Epigenetic targeted therapy of stabilized BAP1 in ASXL1 gain-of-function mutated leukemia. Nat Cancer 2(5):515–526
Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C (2019) MetaDome: Pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat 40(8):1030–1038
Wiesner T, Obenauf AC, Murali R et al (2011) Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 43(10):1018–1021
Wong SJ, Gearhart MD, Taylor AB et al (2016) KDM2B recruitment of the polycomb group complex, PRC1 1, requires cooperation between PCGF1 and BCORL1. Structure 24(10):1795–1801
Xu X, Wells AB, O’Brien DR, Nehorai A, Dougherty JD (2014) Cell type-specific expression analysis to identify putative cellular mechanisms for neurogenetic disorders. J Neurosci 34(4):1420–1431
Yamamoto Y, Abe A, Emi N (2014) Clarifying the impact of polycomb complex component disruption in human cancers impact of bcor and bcorl1 disruption in human cancers. Mol Cancer Res 12(4):479–484
Yamashiro K, Hori K, Lai ES et al (2020) AUTS2 governs cerebellar development, Purkinje cell maturation, motor function and social communication. Iscience 23(12):101820
Yu H, Mashtalir N, Daou S et al (2010) The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 30(21):5071–5085
Zhao W, Hu X, Liu Y et al (2021) A de novo variant of ASXL1 is associated with an atypical phenotype of Bohring-Opitz syndrome: case report and literature review. Front Pediatr. https://doi.org/10.3389/fped.2021.678615
Zhou W, Zhu P, Wang J et al (2008) Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol Cell 29(1):69–80
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human development (R01AWD010411 to SLB), National Institute of Neurological Disorders and Stroke (R01NS101597 to SLB), Leo's Lighthouse Foundation to SLB, NIH Cellular and Molecular Biology Training Grant (T32-GM007315 to CWR), NRSA Fellowship Grant (F31NS127551 to CWR), MSTP (T32GM007863 to CWR), and Neuroscience training grant (T32-NS076401 to ERP).
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human development (R01AWD010411 to SLB), National Institute of Neurological Disorders and Stroke (R01NS101597 to SLB), Leo's Lighthouse Foundation to SLB, NIH Cellular and Molecular Biology Training Grant (T32-GM007315 to CWR), NRSA Fellowship Grant (F31NS127551 to CWR), MSTP (T32GM007863 to CWR), and Neuroscience training grant (T32-NS076401 to ERP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ryan, C.W., Peirent, E.R., Regan, S.L. et al. H2A monoubiquitination: insights from human genetics and animal models. Hum. Genet. 143, 511–527 (2024). https://doi.org/10.1007/s00439-023-02557-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-023-02557-x