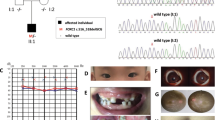
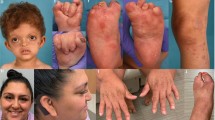
Abstract
Teebi hypertelorism syndrome (THS; OMIM 145420) is a rare craniofacial disorder characterized by hypertelorism, prominent forehead, short nose with broad or depressed nasal root. Some cases of THS have been attributed to SPECC1L variants. Homozygous variants in CDH11 truncating the transmembrane and intracellular domains have been implicated in Elsahy–Waters syndrome (EWS; OMIM 211380) with hypertelorism. We report THS due to CDH11 heterozygous missense variants on 19 subjects from 9 families. All affected residues in the extracellular region of Cadherin-11 (CHD11) are highly conserved across vertebrate species and classical cadherins. Six of the variants that cluster around the EC2–EC3 and EC3–EC4 linker regions are predicted to affect Ca2+ binding that is required for cadherin stability. Two of the additional variants [c.164G > C, p.(Trp55Ser) and c.418G > A, p.(Glu140Lys)] are also notable as they are predicted to directly affect trans-homodimer formation. Immunohistochemical study demonstrates that CDH11 is strongly expressed in human facial mesenchyme. Using multiple functional assays, we show that five variants from the EC1, EC2–EC3 linker, and EC3 regions significantly reduced the cell-substrate trans adhesion activity and one variant from EC3–EC4 linker results in changes in cell morphology, focal adhesion, and migration, suggesting dominant negative effect. Characteristic features in this cohort included depressed nasal root, cardiac and umbilical defects. These features distinguished this phenotype from that seen in SPECC1L-related hypertelorism syndrome and CDH11-related EWS. Our results demonstrate heterozygous variants in CDH11, which decrease cell–cell adhesion and increase cell migratory behavior, cause a form of THS, as termed CDH11-related THS.








Similar content being viewed by others
Data availability
The variants are available in the Leiden Open Variation Database (LOVD, www.LOVD.nl/CDH11).
References
Accogli A, Calabretta S, St-Onge J, Boudrahem-Addour N, Dionne-Laporte A, Joset P, Azzarello-Burri S, Rauch A, Krier J, Fieg E et al (2019) De novo pathogenic variants in N-cadherin cause a syndromic neurodevelopmental disorder with corpus callosum, axon, cardiac, ocular, and genital defects. Am J Hum Genet 105:854–868
Alimperti S, Andreadis ST (2015) CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res 14:270–282
Becker SF, Mayor R, Kashef J (2013) Cadherin-11 mediates contact inhibition of locomotion during Xenopus neural crest cell migration. PLoS ONE 8:e85717
Bhoj EJ, Li D, Harr MH, Tian L, Wang T, Zhao Y, Qiu H, Kim C, Hoffman JD, Hakonarson H et al (2015) Expanding the SPECC1L mutation phenotypic spectrum to include Teebi hypertelorism syndrome. Am J Med Genet A 167A:2497–2502
Bhoj EJ, Haye D, Toutain A, Bonneau D, Nielsen IK, Lund IB, Bogaard P, Leenskjold S, Karaer K, Wild KT et al (2019) Phenotypic spectrum associated with SPECC1L pathogenic variants: new families and critical review of the nosology of Teebi, Opitz GBBB, and Baraitser–Winter syndromes. Eur J Med Genet 62:103588
Biesecker LG, Adam MP, Alkuraya FS, Amemiya AR, Bamshad MJ, Beck AE, Bennett JT, Bird LM, Carey JC, Chung B et al (2021) A dyadic approach to the delineation of diagnostic entities in clinical genomics. Am J Hum Genet 108:8–15
Borchers A, David R, Wedlich D (2001) Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128:3049–3060
Brasch J, Harrison OJ, Honig B, Shapiro L (2012) Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol 22:299–310
Brito LA, Yamamoto GL, Melo S, Malcher C, Ferreira SG, Figueiredo J, Alvizi L, Kobayashi GS, Naslavsky MS, Alonso N et al (2015) Rare variants in the epithelial cadherin gene underlying the genetic etiology of nonsyndromic cleft lip with or without cleft palate. Hum Mutat 36:1029–1033
Castori M, Ott CE, Bisceglia L, Leone MP, Mazza T, Castellana S, Tomassi J, Lanciotti S, Mundlos S, Hennekam RC et al (2018) A novel mutation in CDH11, encoding cadherin-11, cause branchioskeletogenital (Elsahy–Waters) syndrome. Am J Med Genet A 176:2028–2033
Cavey M, Rauzi M, Lenne PF, Lecuit T (2008) A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453:751–756
Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM (2001) Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol 154:231–243
Corso G, Roviello F, Paredes J, Pedrazzani C, Novais M, Correia J, Marrelli D, Cirnes L, Seruca R, Oliveira C et al (2007) Characterization of the P373L E-cadherin germline missense mutation and implication for clinical management. Eur J Surg Oncol 33:1061–1067
Cox LL, Cox TC, Moreno Uribe LM, Zhu Y, Richter CT, Nidey N, Standley JM, Deng M, Blue E, Chong JX et al (2018) Mutations in the epithelial cadherin-p120-catenin complex cause Mendelian non-syndromic cleft lip with or without cleft palate. Am J Hum Genet 102:1143–1157
Esmaeeli Nieh S, Madou MR, Sirajuddin M, Fregeau B, McKnight D, Lexa K, Strober J, Spaeth C, Hallinan BE, Smaoui N et al (2015) De novo mutations in KIF1A cause progressive encephalopathy and brain atrophy. Ann Clin Transl Neurol 2:623–635
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:l1
Ghoumid J, Stichelbout M, Jourdain AS, Frenois F, Lejeune-Dumoulin S, Alex-Cordier MP, Lebrun M, Guerreschi P, Duquennoy-Martinot V, Vinchon M et al (2017) Blepharocheilodontic syndrome is a CDH1 pathway-related disorder due to mutations in CDH1 and CTNND1. Genet Med 19:1013–1021
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE (1998) E-cadherin germline mutations in familial gastric cancer. Nature 392:402–405
Han XD, Cox V, Slavotinek A (2006) Atrioventricular block and wiry hair in Teebi hypertelorism syndrome. Am J Med Genet A 140:1960–1964
Harms FL, Nampoothiri S, Anazi S, Yesodharan D, Alawi M, Kutsche K, Alkuraya FS (2018) Elsahy–Waters syndrome is caused by biallelic mutations in CDH11. Am J Med Genet A 176:477–482
Hoffman JD, Irons M, Schwartz CE, Medne L, Zackai EH (2007) A newly recognized craniosynostosis syndrome with features of Aarskog–Scott and Teebi syndromes. Am J Med Genet A 143A:1282–1286
Huang CF, Lira C, Chu K, Bilen MA, Lee YC, Ye X, Kim SM, Ortiz A, Wu FL, Logothetis CJ et al (2010) Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res 70:4580–4589
Hyafil F, Babinet C, Jacob F (1981) Cell–cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell 26:447–454
Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D (2009) Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev 23:1393–1398
Kawaguchi J, Takeshita S, Kashima T, Imai T, Machinami R, Kudo A (1999) Expression and function of the splice variant of the human cadherin-11 gene in subordination to intact cadherin-11. J Bone Miner Res 14:764–775
Kievit A, Tessadori F, Douben H, Jordens I, Maurice M, Hoogeboom J, Hennekam R, Nampoothiri S, Kayserili H, Castori M et al (2018) Variants in members of the cadherin–catenin complex, CDH1 and CTNND1, cause blepharocheilodontic syndrome. Eur J Hum Genet 26:210–219
Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M (1995) Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol 169:347–358
Kitagawa M, Natori M, Murase S, Hirano S, Taketani S, Suzuki ST (2000) Mutation analysis of cadherin-4 reveals amino acid residues of EC1 important for the structure and function. Biochem Biophys Res Commun 271:358–363
Kjaer KW, Hansen L, Schwabe GC, Marques-de-Faria AP, Eiberg H, Mundlos S, Tommerup N, Rosenberg T (2005) Distinct CDH3 mutations cause ectodermal dysplasia, ectrodactyly, macular dystrophy (EEM syndrome). J Med Genet 42:292–298
Koenig R (2003) Teebi hypertelorism syndrome. Clin Dysmorphol 12:187–189
Kruszka P, Li D, Harr MH, Wilson NR, Swarr D, McCormick EM, Chiavacci RM, Li M, Martinez AF, Hart RA et al (2015) Mutations in SPECC1L, encoding sperm antigen with calponin homology and coiled-coil domains 1-like, are found in some cases of autosomal dominant Opitz G/BBB syndrome. J Med Genet 52:104–110
Langhe RP, Gudzenko T, Bachmann M, Becker SF, Gonnermann C, Winter C, Abbruzzese G, Alfandari D, Kratzer MC, Franz CM et al (2016) Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat Commun 7:10909
Li L, Ying J, Li H, Zhang Y, Shu X, Fan Y, Tan J, Cao Y, Tsao SW, Srivastava G et al (2012) The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/beta-catenin signaling and silenced in common carcinomas. Oncogene 31:3901–3912
Machado-Paula LA, Guion-Almeida ML (2003) Teebi hypertelorism syndrome: additional cases. Am J Med Genet A 117A:181–183
Marchong MN, Yurkowski C, Ma C, Spencer C, Pajovic S, Gallie BL (2010) CDH11 acts as a tumor suppressor in a murine retinoblastoma model by facilitating tumor cell death. PLoS Genet 6:e1000923
Meyer-Schuman R, Antonellis A (2017) Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet 26:R114–R127
Motta M, Fidan M, Bellacchio E, Pantaleoni F, Schneider-Heieck K, Coppola S, Borck G, Salviati L, Zenker M, Cirstea IC et al (2019) Dominant Noonan syndrome-causing LZTR1 mutations specifically affect the Kelch domain substrate-recognition surface and enhance RAS-MAPK signaling. Hum Mol Genet 28:1007–1022
Nabais Sa MJ, Jensik PJ, McGee SR, Parker MJ, Lahiri N, McNeil EP, Kroes HY, Hagerman RJ, Harrison RE, Montgomery T et al (2019) De novo and biallelic DEAF1 variants cause a phenotypic spectrum. Genet Med 21:2059–2069
Nakagawa M, Kondo M, Matsui A (1998) Teebi hypertelorism syndrome with tetralogy of Fallot. Am J Med Genet 77:345–347
Nishimura T, Takeichi M (2009) Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol 89:33–54
Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A, Amann E (1994) Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem 269:12092–12098
Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR et al (2006) Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124:1255–1268
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW (1999) Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res 59:947–952
Richards FM, McKee SA, Rajpar MH, Cole TR, Evans DG, Jankowski JA, McKeown C, Sanders DS, Maher ER (1999) Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet 8:607–610
Saadi I, Alkuraya FS, Gisselbrecht SS, Goessling W, Cavallesco R, Turbe-Doan A, Petrin AL, Harris J, Siddiqui U, Grix AW Jr et al (2011) Deficiency of the cytoskeletal protein SPECC1L leads to oblique facial clefting. Am J Hum Genet 89:44–55
Sobreira N, Schiettecatte F, Valle D, Hamosh A (2015) GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36:928–930
Sprecher E, Bergman R, Richard G, Lurie R, Shalev S, Petronius D, Shalata A, Anbinder Y, Leibu R, Perlman I et al (2001) Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet 29:134–136
Stratton RF (1991) Teebi hypertelorism syndrome (brachycephalofrontonasal dysplasia) in a U.S. family. Am J Med Genet 39:78–80
Takeichi M (1995) Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7:619–627
Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L (1998) Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron 20:1153–1163
Taneyhill LA (2008) To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr 2:223–230
Taskiran EZ, Karaosmanoglu B, Kosukcu C, Dogan OA, Taylan-Sekeroglu H, Simsek-Kiper PO, Utine EG, Boduroglu K, Alikasifoglu M (2017) Homozygous indel mutation in CDH11 as the probable cause of Elsahy–Waters syndrome. Am J Med Genet A 173:3143–3152
Teebi AS (1987) New autosomal dominant syndrome resembling craniofrontonasal dysplasia. Am J Med Genet 28:581–591
Tepass U (1999) Genetic analysis of cadherin function in animal morphogenesis. Curr Opin Cell Biol 11:540–548
Tepass U, Truong K, Godt D, Ikura M, Peifer M (2000) Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 1:91–100
Theveneau E, Mayor R (2012) Cadherins in collective cell migration of mesenchymal cells. Curr Opin Cell Biol 24:677–684
Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA (2000) Cadherin switching in human prostate cancer progression. Cancer Res 60:3650–3654
Toriello HV, Delp K (1994) Teebi hypertelorism syndrome: report of a third family. Clin Dysmorphol 3:335–339
Tsai AC, Robertson JR, Teebi AS (2002) Teebi hypertelorism syndrome: report of a family with previously unrecognized findings. Am J Med Genet 113:302–306
Tsai C, Shi Y, Liu T, Spector E, Roberson J, Meeks N (2018) Loss of function mutation of ACTG1 causes Teebi hyperteorism syndrome in 39th annual David W Smith workshop on malformations and morphogenesis. Banff, Alberta, Canada
Tsukahara M, Uchida M, Shinohara T (1995) Teebi hypertelorism syndrome: further observations. Am J Med Genet 59:59–61
Vunnam N, Pedigo S (2011) Calcium-induced strain in the monomer promotes dimerization in neural cadherin. Biochemistry 50:8437–8444
Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C (2019) MetaDome: pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat 40:1030–1038
Wilson NR, Olm-Shipman AJ, Acevedo DS, Palaniyandi K, Hall EG, Kosa E, Stumpff KM, Smith GJ, Pitstick L, Liao EC et al (2016) SPECC1L deficiency results in increased adherens junction stability and reduced cranial neural crest cell delamination. Sci Rep 6:17735
Yagi T, Takeichi M (2000) Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev 14:1169–1180
Zhang T, Wu Q, Zhu L, Wu D, Yang R, Qi M, Huang X (2020) A novel SPECC1L mutation causing Teebi hypertelorism syndrome: expanding phenotypic and genetic spectrum. Eur J Med Genet 63:103851
Zhu B, Chappuis-Flament S, Wong E, Jensen IE, Gumbiner BM, Leckband D (2003) Functional analysis of the structural basis of homophilic cadherin adhesion. Biophys J 84:4033–4042
Acknowledgements
We thank all the families involved in this study for their participation.
Funding
Research reported in this publication was supported in part by the Roberts Collaborative Functional Genomics Rapid Grant (to D.L., C.S., L.P.) from CHOP. The research conducted at the Murdoch Children’s Research Institute was supported by the Victorian Government’s Operational Infrastructure Support Program. F.B. acknowledges for support and is member of the Italian Undiagnosed Rare Diseases Network led by Dr. Domenica Taruscio (Director, National Centre for Rare Diseases, Istituto Superiore Sanità, Italy). We would like to thank Prof. Ian Glass and Mei Deng, Birth Defects Research Laboratory, University of Washington, for conceptual tissues.
Author information
Authors and Affiliations
Contributions
DL, MB, EPK, TCC, and HH contributed to the molecular evaluation of the affected individuals. LCP, ECB, MSS, OC, KG, AT, APA, LC, BC, CK, AT, BK, CP, MW, TR, EHZ, and EJB contributed to the clinical evaluation of the affected individuals. DL, MEM, PF, LLC, LSM, RM, CS, and FR contributed to the functional investigations. YG, PS, AD, and JC coordinated to the research study subject enrollment. All the authors read, edited, and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interests to declare.
Consent to participate and publish
All the patients’ families from the different institutions agreed to participate and publish in this study and signed appropriate consent forms. The Institutional Review Board of The Children’s Hospital of Philadelphia approved this study. We obtained photo consent for including patients’ photographs from The Children’s Hospital of Philadelphia, Hospital Clínico Universitario Virgen de la Arrixaca, Ghent University Hospital, and Sydney Children’s Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, D., March, M.E., Fortugno, P. et al. Pathogenic variants in CDH11 impair cell adhesion and cause Teebi hypertelorism syndrome. Hum Genet 140, 1061–1076 (2021). https://doi.org/10.1007/s00439-021-02274-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-021-02274-3