Abstract
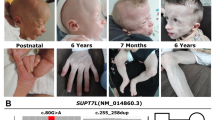
PIGK gene, encoding a key component of glycosylphosphatidylinositol (GPI) transamidase, was recently reported to be associated with inherited GPI deficiency disorders (IGDs). However, little is known about the specific downstream effects of PIGK on neurodevelopment due to the rarity of the disease and the lack of in vivo study. Here, we described 2 patients in a Chinese family presented with profound global developmental delay, severe hypotonia, seizures, and postnatal progressive global brain atrophy including hemisphere, cerebellar and corpus callosum atrophy. Two novel compound heterozygous variants in PIGK were identified via genetic analysis, which was proved to cause significant decrease of PIGK protein and reduced cell surface presence of GPI-APs in the patients. To explore the role of Pigk on embryonic and neuronal development, we constructed Pigk knock-down zebrafish and knock-in mouse models. Zebrafish injected with a small dose of morpholino oligonucleotides displayed severe developmental defects including small eyes, deformed head, curly spinal cord, and unconsumed yolk sac. Primary motor neuronal dysplasia and extensive neural cell apoptosis were further observed. Meanwhile, the mouse models, carrying the two variants respectively homologous with the patients, both resulted in complete embryonic lethality of the homozygotes, which suggested the intolerable effect caused by amino acid substitution of Asp204 as well as the truncated mutation. Our findings provide the in vivo evidence for the essential role of PIGK during the embryonic and neuronal development. Based on these data, we propose a basis for further study of pathological and molecular mechanisms of PIGK-related neurodevelopmental defects.






Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available upon reasonable request.
References
Baratang NV, Jimenez Cruz DA, Ajeawung NF, Nguyen TTM, Pacheco-Cuéllar G, Campeau PM (2019) Inherited glycophosphatidylinositol deficiency variant database and analysis of pathogenic variants. Mol Genet Genomic Med 7(7):e00743. https://doi.org/10.1002/mgg3.743
Bellai-Dussault K, Nguyen TTM, Baratang NV, Jimenez-Cruz DA, Campeau PM (2019) Clinical variability in inherited glycosylphosphatidylinositol deficiency disorders. Clin Genet 95(1):112–121. https://doi.org/10.1111/cge.13425
Benghezal M, Benachour A, Rusconi S, Aebi M, Conzelmann A (1996) Yeast Gpi8p is essential for GPI anchor attachment onto proteins. EMBO J 15(23):6575–6583. https://doi.org/10.1002/j.1460-2075.1996.tb01048.x
Bervoets I, Charlier D (2019) Diversity, versatility and complexity of bacterial gene regulation mechanisms: opportunities and drawbacks for applications in synthetic biology. FEMS Microbiol Rev 43(3):304–339. https://doi.org/10.1093/femsre/fuz001
Carmean V, Yonkers MA, Tellez MB, Willer JR, Willer GB, Gregg RG, Geisler R, Neuhauss SC, Ribera AB (2015) pigk Mutation underlies macho behavior and affects Rohon-Beard cell excitability. J Neurophysiol 114(2):1146–1157. https://doi.org/10.1152/jn.00355.2015
Carmody LC, Blau H, Danis D, Zhang XA, Gourdine J-P, Vasilevsky N, Krawitz P, Thompson MD, Robinson PN (2020) Significantly different clinical phenotypes associated with mutations in synthesis and transamidase+remodeling glycosylphosphatidylinositol (GPI)-anchor biosynthesis genes. Orphanet J Rare Dis 15(1):40. https://doi.org/10.1186/s13023-020-1313-0
Cenik C, Cenik ES, Byeon GW, Grubert F, Candille SI, Spacek D, Alsallakh B, Tilgner H, Araya CL, Tang H, Ricci E, Snyder MP (2015) Integrative analysis of RNA, translation, and protein levels reveals distinct regulatory variation across humans. Genome Res 25(11):1610–1621. https://doi.org/10.1101/gr.193342.115
Diep DB, Nelson KL, Raja SM, Pleshak EN, Buckley JT (1998) Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem 273(4):2355–2360. https://doi.org/10.1074/jbc.273.4.2355
Fan Y, Yin W, Hu B, Kline AD, Zhang VW, Liang D, Sun Y, Wang L, Tang S, Powis Z, Li L, Yan H, Shi Z, Yang X, Chen Y, Wang J, Jiang Y, Tan H, Gu X, Wu L, Yu Y (2018) De Novo Mutations of CCNK cause a syndromic neurodevelopmental disorder with distinctive facial dysmorphism. Am J Hum Genet 103(3):448–455. https://doi.org/10.1016/j.ajhg.2018.07.019
Kinoshita T (2020) Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol 10(3):190290. https://doi.org/10.1098/rsob.190290
Kinoshita T, Fujita M (2016) Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J Lipid Res 57:1. https://doi.org/10.1194/jlr.R063313
Kinoshita T, Inoue N (2000) Dissecting and manipulating the pathway for glycosylphos-phatidylinositol-anchor biosynthesis. Curr Opin Chem Biol 4(6):632–638. https://doi.org/10.1016/s1367-5931(00)00151-4
Knaus A, Kortüm F, Kleefstra T, Stray-Pedersen A, Đukić D, Murakami Y, Gerstner T, van Bokhoven H, Iqbal Z, Horn D, Kinoshita T, Hempel M, Krawitz PM (2019) Mutations in PIGU impair the function of the GPI transamidase complex, causing severe intellectual disability, epilepsy, and brain anomalies. Am J Hum Genet 105(2):395–402. https://doi.org/10.1016/j.ajhg.2019.06.009
Kurosaki T, Maquat LE (2016) Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci 129(3):461–467. https://doi.org/10.1242/jcs.181008
Kvarnung M, Nilsson D, Lindstrand A, Korenke GC, Chiang SCC, Blennow E, Bergmann M, Stödberg T, Mäkitie O, Anderlid B-M, Bryceson YT, Nordenskjöld M, Nordgren A (2013) A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet 50(8):521–528. https://doi.org/10.1136/jmedgenet-2013-101654
Lam C, Golas GA, Davids M, Huizing M, Kane MS, Krasnewich DM, Malicdan MCV, Adams DR, Markello TC, Zein WM, Gropman AL, Lodish MB, Stratakis CA, Maric I, Rosenzweig SD, Baker EH, Ferreira CR, Danylchuk NR, Kahler S, Garnica AD, Bradley Schaefer G, Boerkoel CF, Gahl WA, Wolfe LA (2015) Expanding the clinical and molecular characteristics of PIGT-CDG, a disorder of glycosylphosphatidylinositol anchors. Mol Genet Metab 115(2–3):128–140. https://doi.org/10.1016/j.ymgme.2015.04.007
Li J, Shi L, Zhang K, Zhang Y, Hu S, Zhao T, Teng H, Li X, Jiang Y, Ji L, Sun Z (2018) VarCards: an integrated genetic and clinical database for coding variants in the human genome. Nucleic Acids Res 46(D1):D1039–D1048. https://doi.org/10.1093/nar/gkx1039
Meyer U, Benghezal M, Imhof I, Conzelmann A (2000) Active site determination of Gpi8p, a caspase-related enzyme required for glycosylphosphatidylinositol anchor addition to proteins. Biochemistry 39(12):3461–3471. https://doi.org/10.1021/bi992186o
Mohney RP, Knez JJ, Ravi L, Sevlever D, Rosenberry TL, Hirose S, Medof ME (1994) Glycoinositol phospholipid anchor-defective K562 mutants with biochemical lesions distinct from those in Thy-1- murine lymphoma mutants. J Biol Chem 269(9):6536–6542. https://doi.org/10.1007/BF00023986
Nakashima M, Kashii H, Murakami Y, Kato M, Tsurusaki Y, Miyake N, Kubota M, Kinoshita T, Saitsu H, Matsumoto N (2014) Novel compound heterozygous PIGT mutations caused multiple congenital anomalies-hypotonia-seizures syndrome 3. Neurogenetics 15(3):193–200. https://doi.org/10.1007/s10048-014-0408-y
Nguyen TTM, Murakami Y, Wigby KM, Baratang NV, Rousseau J, St-Denis A, Rosenfeld JA, Laniewski SC, Jones J, Iglesias AD, Jones MC, Masser-Frye D, Scheuerle AE, Perry DL, Taft RJ, Le Deist F, Thompson M, Kinoshita T, Campeau PM (2018) Mutations in PIGS, encoding a GPI transamidase, cause a neurological syndrome ranging from fetal akinesia to epileptic encephalopathy. Am J Hum Genet 103(4):602–611. https://doi.org/10.1016/j.ajhg.2018.08.014
Nguyen TTM, Murakami Y, Mobilio S, Niceta M, Zampino G, Philippe C, Moutton S, Zaki MS, James KN, Musaev D, Mu W, Baranano K, Nance JR, Rosenfeld JA, Braverman N, Ciolfi A, Millan F, Person RE, Bruel A-L, Thauvin-Robinet C, Ververi A, DeVile C, Male A, Efthymiou S, Maroofian R, Houlden H, Maqbool S, Rahman F, Baratang NV, Rousseau J, St-Denis A, Elrick MJ, Anselm I, Rodan LH, Tartaglia M, Gleeson J, Kinoshita T, Campeau PM (2020) Bi-allelic variants in the GPI transamidase subunit PIGK cause a neurodevelopmental syndrome with hypotonia, cerebellar atrophy, and epilepsy. Am J Hum Genet 106(4):484–495. https://doi.org/10.1016/j.ajhg.2020.03.001
Ohishi K, Inoue N, Maeda Y, Takeda J, Riezman H, Kinoshita T (2000) Gaa1p and gpi8p are components of a glycosylphosphatidylinositol (GPI) transamidase that mediates attachment of GPI to proteins. Mol Biol Cell 11(5):1523–1533. https://doi.org/10.1091/mbc.11.5.1523
Ohishi K, Nagamune K, Maeda Y, Kinoshita T (2003) Two subunits of glycosylphosphatidylinositol transamidase, GPI8 and PIG-T, form a functionally important intermolecular disulfide bridge. J Biol Chem 278(16):13959–13967. https://doi.org/10.1074/jbc.M300586200
Pagnamenta AT, Murakami Y, Taylor JM, Anzilotti C, Howard MF, Miller V, Johnson DS, Tadros S, Mansour S, Temple IK, Firth R, Rosser E, Harrison RE, Kerr B, Popitsch N, Kinoshita T, Taylor JC, Kini U (2017) Analysis of exome data for 4293 trios suggests GPI-anchor biogenesis defects are a rare cause of developmental disorders. Eur J Human Genetics EJHG 25(6):669–679. https://doi.org/10.1038/ejhg.2017.32
Price AM, Dai J, Bazot Q, Patel L, Nikitin PA, Djavadian R, Winter PS, Salinas CA, Barry AP, Wood KC, Johannsen EC, Letai A, Allday MJ, Luftig MA (2017) Epstein-Barr virus ensures B cell survival by uniquely modulating apoptosis at early and late times after infection. Elife. https://doi.org/10.7554/eLife.22509
Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S-i (2009) Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci 29(21):6780–6793. https://doi.org/10.1523/JNEUROSCI.0801-09.2009
Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. https://doi.org/10.1038/nature10098
Ścieżyńska A, Ruszkowska E, Szulborski K, Rydz K, Wierzbowska J, Kosińska J, Rękas M, Płoski R, Szaflik JP, Ołdak M (2017) Processing of OPA1 with a novel N-terminal mutation in patients with autosomal dominant optic atrophy: Escape from nonsense-mediated decay. PLoS ONE 12(8):e0183866. https://doi.org/10.1371/journal.pone.0183866
Shamseldin HE, Tulbah M, Kurdi W, Nemer M, Alsahan N, Al Mardawi E, Khalifa O, Hashem A, Kurdi A, Babay Z, Bubshait DK, Ibrahim N, Abdulwahab F, Rahbeeni Z, Hashem M, Alkuraya FS (2015) Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. https://doi.org/10.1186/s13059-015-0681-6
Udenfriend S, Kodukula K (1995) How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. https://doi.org/10.1146/annurev.bi.64.070195.003023
Westerfield M (2000) The zebrafish book : a guide for the laboratory use of zebrafish (Danio rerio). University of Oregon Press, Eugene, OR
Wilbe M, Ekvall S, Eurenius K, Ericson K, Casar-Borota O, Klar J, Dahl N, Ameur A, Annerén G, Bondeson M-L (2015) MuSK: a new target for lethal fetal akinesia deformation sequence (FADS). J Med Genet 52(3):195–202. https://doi.org/10.1136/jmedgenet-2014-102730
Yin W, Hu B (2014) Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish. Exp Neurol. https://doi.org/10.1016/j.expneurol.2013.11.012
Yu J, Nagarajan S, Knez JJ, Udenfriend S, Chen R, Medof ME (1997) The affected gene underlying the class K glycosylphosphatidylinositol (GPI) surface protein defect codes for the GPI transamidase. Proc Natl Acad Sci USA 94(23):12580–12585. https://doi.org/10.1073/pnas.94.23.12580
Acknowledgments
We acknowledge the generous support from the families included in this article. We are grateful to Dr. Hu Tan for help with case data collected and Dr. Lanlan Zeng for useful experimental advice.
Funding
This work was supported by the grants from the National Key R&D Program of China (2018YFC1002201, 2017YFC1001802, 2019YFA0405603), the National Natural Science Foundation of China (81974240, 81970829, 81771599), the Major Scientific and Technological Projects for Collaborative Prevention and Control of Birth Defects in Hunan Province (2019SK1010, 2019SK1014) and the Key Research and Development Plan Program of Anhui Province (202004j07020020).
Author information
Authors and Affiliations
Contributions
XC, WY, ZL, BH and LW designed the study. XC and YT provided patients’ data and performed clinical assessments. XC, WY, SC and WZ conducted experiments. HL, HK, MZ, JZ and GS participated in data analysis. XC, WY, DL, ZL and LW co-wrote the manuscript. DL, ZL, BH and LW supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Research Ethics Committee of Hunan Jiahui Genetics Hospital. The animal manipulations were conducted in strict accordance with the guidelines and regulations set forth by Central South University (CSU) Animal Resources Center and University Animal Care and Use Committee and University of Science and Technology of China (USTC) Animal Resources Center and University Animal Care and Use Committee respectively.
Consent to participate
Written informed consent was obtained from the parents.
Consent for publication
The participants have consented to the submission of the article to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 334 KB)
Rights and permissions
About this article
Cite this article
Chen, X., Yin, W., Chen, S. et al. Loss of PIGK function causes severe infantile encephalopathy and extensive neuronal apoptosis. Hum Genet 140, 791–803 (2021). https://doi.org/10.1007/s00439-020-02243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02243-2