Abstract
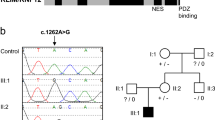
PRR12 encodes a proline-rich protein nuclear factor suspected to be involved in neural development. Its nuclear expression in fetal brains and in the vision system supports its role in brain and eye development more specifically. However, its function and potential role in human disease has not been determined. Recently, a de novo t(10;19) (q22.3;q13.33) translocation disrupting the PRR12 gene was detected in a girl with intellectual disability and neuropsychiatric alterations. Here we report on three unrelated patients with heterozygous de novo apparent loss-of-function mutations in PRR12 detected by clinical whole exome sequencing: c.1918G>T (p.Glu640*), c.4502_4505delTGCC (p.Leu1501Argfs*146) and c.903_909dup (p.Pro304Thrfs*46). All three patients had global developmental delay, intellectual disability, eye and vision abnormalities, dysmorphic features, and neuropsychiatric problems. Eye abnormalities were consistent among the three patients and consisted of stellate iris pattern and iris coloboma. Additional variable clinical features included hypotonia, skeletal abnormalities, sleeping problems, and behavioral issues such as autism and anxiety. In summary, we propose that haploinsufficiency of PRR12 is associated with this novel multisystem neurodevelopmental disorder.


Similar content being viewed by others
References
Choudhary C, Kumar C, Gnad F et al (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325:834–840
Cordova-Fletes C, Dominguez MG, Delint-Ramirez I et al (2015) A de novo t(10;19)(q22.3;q13.33) leads to ZMIZ1/PRR12 reciprocal fusion transcripts in a girl with intellectual disability and neuropsychiatric alterations. Neurogenetics 16:287–298
Deciphering Developmental Disorders S (2015) Large-scale discovery of novel genetic causes of developmental disorders. Nature 519:223–228
Dephoure N, Zhou C, Villen J et al (2008) A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105:10762–10767
Gardiner-Garden M, Frommer M (1987) CpG islands in vertebrate genomes. J Mol Biol 196:261–282
Hao YH, Fountain MD Jr, Fon Tacer K et al (2015) USP7 Acts as a molecular rheostat to promote WASH-dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Mol Cell 59:956–969
Havugimana PC, Hart GT, Nepusz T et al (2012) A census of human soluble protein complexes. Cell 150:1068–1081
Hein MY, Hubner NC, Poser I et al (2015) A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163:712–723
Lek M, Karczewski KJ, Minikel EV et al (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536:285–291
Li J, Zhao G, Gao X (2013) Development of neurodevelopmental disorders: a regulatory mechanism involving bromodomain-containing proteins. J Neurodev Disord 5:4
Nakamura KM, Diehl NN, Mohney BG (2011) Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol 129:69–74
Olsen JV, Blagoev B, Gnad F et al (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648
Olsen JV, Vermeulen M, Santamaria A et al (2010) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3:3
Wang J, Huo K, Ma L et al (2011) Toward an understanding of the protein interaction network of the human liver. Mol Syst Biol 7:536
Yang Y, Muzny DM, Reid JG et al (2013) Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med 369:1502–1511
Yang Y, Muzny DM, Xia F et al (2014) Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312:1870–1879
Acknowledgements
We thank the patients and families for their participation in this study. Magalie Leduc, Yaping Yang, Christine Eng and Weimin Bi are paid employees of Baylor Genetics. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from molecular genetic testing offered in the Baylor Genetics Laboratories. The DDD study presents independent research commissioned by the Health Innovation Challenge Fund (Grant number HICF-1009-003), a parallel funding partnership between the Wellcome Trust and the Department of Health, and the Wellcome Trust Sanger Institute (Grant number WT098051). The views expressed in this publication are those of the authors and not necessarily those of the Wellcome Trust or the Department of Health. The research team acknowledges the support of the National Institute for Health Research, through the Comprehensive Clinical Research Network.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leduc, M.S., Mcguire, M., Madan-Khetarpal, S. et al. De novo apparent loss-of-function mutations in PRR12 in three patients with intellectual disability and iris abnormalities. Hum Genet 137, 257–264 (2018). https://doi.org/10.1007/s00439-018-1877-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-018-1877-0