Abstract
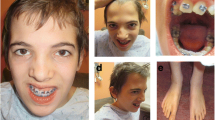
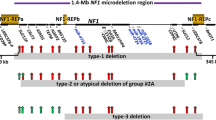
Hereditary neuropathy with liability to pressure palsies (HNPP) and Smith–Magenis syndrome (SMS) are genomic disorders associated with deletion copy number variants involving chromosome 17p12 and 17p11.2, respectively. Nonallelic homologous recombination (NAHR)-mediated recurrent deletions are responsible for the majority of HNPP and SMS cases; the rearrangement products encompass the key dosage-sensitive genes PMP22 and RAI1, respectively, and result in haploinsufficiency for these genes. Less frequently, nonrecurrent genomic rearrangements occur at this locus. Contiguous gene duplications encompassing both PMP22 and RAI1, i.e., PMP22-RAI1 duplications, have been investigated, and replication-based mechanisms rather than NAHR have been proposed for these rearrangements. In the current study, we report molecular and clinical characterizations of six subjects with the reciprocal phenomenon of deletions spanning both genes, i.e., PMP22-RAI1 deletions. Molecular studies utilizing high-resolution array comparative genomic hybridization and breakpoint junction sequencing identified mutational signatures that were suggestive of replication-based mechanisms. Systematic clinical studies revealed features consistent with SMS, including features of intellectual disability, speech and gross motor delays, behavioral problems and ocular abnormalities. Five out of six subjects presented clinical signs and/or objective electrophysiologic studies of peripheral neuropathy. Clinical profiling may improve the clinical management of this unique group of subjects, as the peripheral neuropathy can be more severe or of earlier onset as compared to SMS patients having the common recurrent deletion. Moreover, the current study, in combination with the previous report of PMP22-RAI1 duplications, contributes to the understanding of rare complex phenotypes involving multiple dosage-sensitive genes from a genetic mechanistic standpoint.



Similar content being viewed by others
References
Bai Y, Zhang X, Katona I, Saporta MA, Shy ME, O’Malley HA, Isom LL, Suter U, Li J (2010) Conduction block in PMP22 deficiency. J Neurosci 30:600–608. doi:10.1523/JNEUROSCI.4264-09.2010
Ballabio A, Bardoni B, Carrozzo R, Andria G, Bick D, Campbell L, Hamel B, Ferguson-Smith MA, Gimelli G, Fraccaro M et al (1989) Contiguous gene syndromes due to deletions in the distal short arm of the human X chromosome. Proc Natl Acad Sci USA 86:10001–10005
Bi W, Yan J, Stankiewicz P, Park SS, Walz K, Boerkoel CF, Potocki L, Shaffer LG, Devriendt K, Nowaczyk MJ, Inoue K, Lupski JR (2002) Genes in a refined Smith–Magenis syndrome critical deletion interval on chromosome 17p11.2 and the syntenic region of the mouse. Genome Res 12:713–728. doi:10.1101/gr.73702
Bi W, Saifi GM, Shaw CJ, Walz K, Fonseca P, Wilson M, Potocki L, Lupski JR (2004) Mutations of RAI1, a PHD-containing protein, in nondeletion patients with Smith-Magenis syndrome. Hum Genet 115:515–524. doi:10.1007/s00439-004-1187-6
Bi W, Saifi GM, Girirajan S, Shi X, Szomju B, Firth H, Magenis RE, Potocki L, Elsea SH, Lupski JR (2006) RAI1 point mutations, CAG repeat variation, and SNP analysis in non-deletion Smith–Magenis syndrome. Am J Med Genet A 140:2454–2463. doi:10.1002/ajmg.a.31510
Boone PM, Liu P, Zhang F, Carvalho CM, Towne CF, Batish SD, Lupski JR (2011) Alu-specific microhomology-mediated deletion of the final exon of SPAST in three unrelated subjects with hereditary spastic paraplegia. Genet Med 13:582–592. doi:10.1097/GIM.0b013e3182106775
Boone PM, Yuan B, Campbell IM, Scull JC, Withers MA, Baggett BC, Beck CR, Shaw CJ, Stankiewicz P, Moretti P, Goodwin WE, Hein N, Fink JK, Seong MW, Seo SH, Park SS, Karbassi ID, Batish SD, Ordonez-Ugalde A, Quintans B, Sobrido MJ, Stemmler S, Lupski JR (2014) The Alu-rich genomic architecture of SPAST predisposes to diverse and functionally distinct disease-associated CNV alleles. Am J Hum Genet 95:143–161. doi:10.1016/j.ajhg.2014.06.014
Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D (1998) A chromosomal deletion map of human malformations. Am J Hum Genet 63:1153–1159. doi:10.1086/302041
Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D (1999) A chromosomal duplication map of malformations: regions of suspected haplo- and triplolethality–and tolerance of segmental aneuploidy–in humans. Am J Hum Genet 64:1702–1708. doi:10.1086/302410
Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR (1994) Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease–a contiguous gene syndrome. Nat Genet 8:328–332. doi:10.1038/ng1294-328
Campbell IM, Yatsenko SA, Hixson P, Reimschisel T, Thomas M, Wilson W, Dayal U, Wheless JW, Crunk A, Curry C, Parkinson N, Fishman L, Riviello JJ, Nowaczyk MJ, Zeesman S, Rosenfeld JA, Bejjani BA, Shaffer LG, Cheung SW, Lupski JR, Stankiewicz P, Scaglia F (2012) Novel 9q34.11 gene deletions encompassing combinations of four Mendelian disease genes: STXBP1, SPTAN1, ENG, and TOR1A. Genet Med 14:868–876. doi:10.1038/gim.2012.65
Campbell IM, Yuan B, Robberecht C, Pfundt R, Szafranski P, McEntagart ME, Nagamani SC, Erez A, Bartnik M, Wisniowiecka-Kowalnik B, Plunkett KS, Pursley AN, Kang SH, Bi W, Lalani SR, Bacino CA, Vast M, Marks K, Patton M, Olofsson P, Patel A, Veltman JA, Cheung SW, Shaw CA, Vissers LE, Vermeesch JR, Lupski JR, Stankiewicz P (2014) Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am J Hum Genet 95:173–182. doi:10.1016/j.ajhg.2014.07.003
Carvalho CM, Lupski JR (2016) Mechanisms underlying structural variant formation in genomic disorders. Nat Rev Genet 17:224–238. doi:10.1038/nrg.2015.25
Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, Swanson PD, Odelberg SJ, Disteche CM, Bird TD (1993) DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 72:143–151
Chen KS, Manian P, Koeuth T, Potocki L, Zhao Q, Chinault AC, Lee CC, Lupski JR (1997) Homologous recombination of a flanking repeat gene cluster is a mechanism for a common contiguous gene deletion syndrome. Nat Genet 17:154–163. doi:10.1038/ng1097-154
Chrestian N, McMillan H, Poulin C, Campbell C, Vajsar J (2015) Hereditary neuropathy with liability to pressure palsies in childhood: case series and literature update. Neuromuscul Disord 25:693–698. doi:10.1016/j.nmd.2015.06.463
De Jong JGY (1947) Over families met hereditarie disposite tot het optreten van neuritiden, gecorreleard met migraine. Psychiat Neurol Bl 50:60–76
Dittwald P, Gambin T, Szafranski P, Li J, Amato S, Divon MY, Rodriguez Rojas LX, Elton LE, Scott DA, Schaaf CP, Torres-Martinez W, Stevens AK, Rosenfeld JA, Agadi S, Francis D, Kang SH, Breman A, Lalani SR, Bacino CA, Bi W, Milosavljevic A, Beaudet AL, Patel A, Shaw CA, Lupski JR, Gambin A, Cheung SW, Stankiewicz P (2013) NAHR-mediated copy-number variants in a clinical population: mechanistic insights into both genomic disorders and Mendelizing traits. Genome Res 23:1395–1409. doi:10.1101/gr.152454.112
Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, Elsea SH (2007) Gender, genotype, and phenotype differences in Smith–Magenis syndrome: a meta-analysis of 105 cases. Clin Genet 71:540–550. doi:10.1111/j.1399-0004.2007.00815.x
Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, Bibillo A, Bjornson K, Chaudhuri B, Christians F, Cicero R, Clark S, Dalal R, Dewinter A, Dixon J, Foquet M, Gaertner A, Hardenbol P, Heiner C, Hester K, Holden D, Kearns G, Kong X, Kuse R, Lacroix Y, Lin S, Lundquist P, Ma C, Marks P, Maxham M, Murphy D, Park I, Pham T, Phillips M, Roy J, Sebra R, Shen G, Sorenson J, Tomaney A, Travers K, Trulson M, Vieceli J, Wegener J, Wu D, Yang A, Zaccarin D, Zhao P, Zhong F, Korlach J, Turner S (2009) Real-time DNA sequencing from single polymerase molecules. Science 323:133–138. doi:10.1126/science.1162986
English AC, Salerno WJ, Hampton OA, Gonzaga-Jauregui C, Ambreth S, Ritter DI, Beck CR, Davis CF, Dahdouli M, Ma S, Carroll A, Veeraraghavan N, Bruestle J, Drees B, Hastie A, Lam ET, White S, Mishra P, Wang M, Han Y, Zhang F, Stankiewicz P, Wheeler DA, Reid JG, Muzny DM, Rogers J, Sabo A, Worley KC, Lupski JR, Boerwinkle E, Gibbs RA (2015) Assessing structural variation in a personal genome-towards a human reference diploid genome. BMC Genom 16:286. doi:10.1186/s12864-015-1479-3
Gabreels-Festen AA, Gabreels FJ, Joosten EM, Vingerhoets HM, Renier WO (1992) Hereditary neuropathy with liability to pressure palsies in childhood. Neuropediatrics 23:138–143
Girirajan S, Vlangos CN, Szomju BB, Edelman E, Trevors CD, Dupuis L, Nezarati M, Bunyan DJ, Elsea SH (2006) Genotype-phenotype correlation in Smith–Magenis syndrome: evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genet Med 8:417–427. doi:10.1097/01.gim.0000228215.32110.89
Goh ES, Banwell B, Stavropoulos DJ, Shago M, Yoon G (2014) Mosaic microdeletion of 17p11.2-p12 and duplication of 17q22-q24 in a girl with Smith–Magenis phenotype and peripheral neuropathy. Am J Med Genet A 164A:748–752. doi:10.1002/ajmg.a.36322
Greenberg F, Guzzetta V, Montes de Oca-Luna R, Magenis RE, Smith AC, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR (1991) Molecular analysis of the Smith–Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2). Am J Hum Genet 49:1207–1218
Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown F, Dutton R, McCluggage C, Friedman E, Sulek M, Lupski JR (1996) Multi-disciplinary clinical study of Smith–Magenis syndrome (deletion 17p11.2). Am J Med Genet 62:247–254. doi:10.1002/(SICI)1096-8628(19960329)62:3<247:AID-AJMG9>3.0.CO;2-Q
Hastings PJ, Ira G, Lupski JR (2009) A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet 5:e1000327. doi:10.1371/journal.pgen.1000327
Juyal RC, Figuera LE, Hauge X, Elsea SH, Lupski JR, Greenberg F, Baldini A, Patel PI (1996) Molecular analyses of 17p11.2 deletions in 62 Smith–Magenis syndrome patients. Am J Hum Genet 58:998–1007
Lacaria M, Saha P, Potocki L, Bi W, Yan J, Girirajan S, Burns B, Elsea S, Walz K, Chan L, Lupski JR, Gu W (2012) A duplication CNV that conveys traits reciprocal to metabolic syndrome and protects against diet-induced obesity in mice and men. PLoS Genet 8:e1002713. doi:10.1371/journal.pgen.1002713
Lee JA, Carvalho CM, Lupski JR (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131:1235–1247. doi:10.1016/j.cell.2007.11.037
Li J, Ghandour K, Radovanovic D, Shy RR, Krajewski KM, Shy ME, Nicholson GA (2007) Stoichiometric alteration of PMP22 protein determines the phenotype of hereditary neuropathy with liability to pressure palsies. Arch Neurol 64:974–978. doi:10.1001/archneur.64.7.974
Liburd N, Ghosh M, Riazuddin S, Naz S, Khan S, Ahmed Z, Riazuddin S, Liang Y, Menon PS, Smith T, Smith AC, Chen KS, Lupski JR, Wilcox ER, Potocki L, Friedman TB (2001) Novel mutations of MYO15A associated with profound deafness in consanguineous families and moderately severe hearing loss in a patient with Smith–Magenis syndrome. Hum Genet 109:535–541. doi:10.1007/s004390100604
Liu P, Lacaria M, Zhang F, Withers M, Hastings PJ, Lupski JR (2011) Frequency of nonallelic homologous recombination is correlated with length of homology: evidence that ectopic synapsis precedes ectopic crossing-over. Am J Hum Genet 89:580–588. doi:10.1016/j.ajhg.2011.09.009
Lupianez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, Horn D, Kayserili H, Opitz JM, Laxova R, Santos-Simarro F, Gilbert-Dussardier B, Wittler L, Borschiwer M, Haas SA, Osterwalder M, Franke M, Timmermann B, Hecht J, Spielmann M, Visel A, Mundlos S (2015) Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161:1012–1025. doi:10.1016/j.cell.2015.04.004
Lupski JR (1998) Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 14:417–422
Lupski JR (2009) Genomic disorders ten years on. Genome Med 1:42. doi:10.1186/gm42
Lupski JR (2015) Structural variation mutagenesis of the human genome: impact on disease and evolution. Environ Mol Mutagen 56:419–436. doi:10.1002/em.21943
Lupski JR, Stankiewicz P (2005) Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 1:e49. doi:10.1371/journal.pgen.0010049
Mayle R, Campbell IM, Beck CR, Yu Y, Wilson M, Shaw CA, Bjergbaek L, Lupski JR, Ira G (2015) DNA REPAIR. Mus81 and converging forks limit the mutagenicity of replication fork breakage. Science 349:742–747. doi:10.1126/science.aaa8391
McVey M, Lee SE (2008) MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet 24:529–538. doi:10.1016/j.tig.2008.08.007
Neira-Fresneda J, Potocki L (2015) Neurodevelopmental Disorders Associated with Abnormal Gene Dosage: Smith–Magenis and Potocki-Lupski syndromes. J Pediatric Genetics 04:159–167. doi:10.1055/s-0035-1564443
Page SL, Shin JC, Han JY, Choo KH, Shaffer LG (1996) Breakpoint diversity illustrates distinct mechanisms for Robertsonian translocation formation. Hum Mol Genet 5:1279–1288
Pentao L, Wise CA, Chinault AC, Patel PI, Lupski JR (1992) Charcot-Marie-Tooth type 1A duplication appears to arise from recombination at repeat sequences flanking the 1.5 Mb monomer unit. Nat Genet 2:292–300. doi:10.1038/ng1292-292
Potocki L, Chen KS, Koeuth T, Killian J, Iannaccone ST, Shapira SK, Kashork CD, Spikes AS, Shaffer LG, Lupski JR (1999) DNA rearrangements on both homologues of chromosome 17 in a mildly delayed individual with a family history of autosomal dominant carpal tunnel syndrome. Am J Hum Genet 64:471–478. doi:10.1086/302240
Ricard G, Molina J, Chrast J, Gu W, Gheldof N, Pradervand S, Schutz F, Young JI, Lupski JR, Reymond A, Walz K (2010) Phenotypic consequences of copy number variation: insights from Smith–Magenis and Potocki-Lupski syndrome mouse models. PLoS Biol 8:e1000543. doi:10.1371/journal.pbio.1000543
Segal Y, Peissel B, Renieri A, de Marchi M, Ballabio A, Pei Y, Zhou J (1999) LINE-1 elements at the sites of molecular rearrangements in Alport syndrome-diffuse leiomyomatosis. Am J Hum Genet 64:62–69. doi:10.1086/302213
Shaw CJ, Lupski JR (2005) Non-recurrent 17p11.2 deletions are generated by homologous and non-homologous mechanisms. Hum Genet 116:1–7. doi:10.1007/s00439-004-1204-9
Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH (2003) Mutations in RAI1 associated with Smith–Magenis syndrome. Nat Genet 33:466–468. doi:10.1038/ng1126
Staal A, de Weerdt CJ, Went LN (1965) Hereditary compression syndrome of peripheral nerves. Neurology 15:1008–1017
Stankiewicz P, Lupski JR (2010) Structural variation in the human genome and its role in disease. Annu Rev Med 61:437–455. doi:10.1146/annurev-med-100708-204735
Stankiewicz P, Shaw CJ, Dapper JD, Wakui K, Shaffer LG, Withers M, Elizondo L, Park SS, Lupski JR (2003) Genome architecture catalyzes nonrecurrent chromosomal rearrangements. Am J Hum Genet 72:1101–1116. doi:10.1086/374385
Szafranski P, Gambin T, Dharmadhikari AV, Akdemir KC, Jhangiani SN, Schuette J, Godiwala N, Yatsenko SA, Sebastian J, Madan-Khetarpal S, Surti U, Abellar RG, Bateman DA, Wilson AL, Markham MH, Slamon J, Santos-Simarro F, Palomares M, Nevado J, Lapunzina P, Chung BH, Wong WL, Chu YW, Mok GT, Kerem E, Reiter J, Ambalavanan N, Anderson SA, Kelly DR, Shieh J, Rosenthal TC, Scheible K, Steiner L, Iqbal MA, McKinnon ML, Hamilton SJ, Schlade-Bartusiak K, English D, Hendson G, Roeder ER, DeNapoli TS, Littlejohn RO, Wolff DJ, Wagner CL, Yeung A, Francis D, Fiorino EK, Edelman M, Fox J, Hayes DA, Janssens S, De Baere E, Menten B, Loccufier A, Vanwalleghem L, Moerman P, Sznajer Y, Lay AS, Kussmann JL, Chawla J, Payton DJ, Phillips GE, Brosens E, Tibboel D, de Klein A, Maystadt I, Fisher R, Sebire N, Male A, Chopra M, Pinner J, Malcolm G, Peters G, Arbuckle S, Lees M, Mead Z, Quarrell O, Sayers R, Owens M, Shaw-Smith C, Lioy J, McKay E, de Leeuw N, Feenstra I, Spruijt L, Elmslie F, Thiruchelvam T, Bacino CA, Langston C, Lupski JR, Sen P, Popek E, Stankiewicz P (2016) Pathogenetics of alveolar capillary dysplasia with misalignment of pulmonary veins. Hum Genet. doi:10.1007/s00439-016-1655-9
Trask BJ, Mefford H, van den Engh G, Massa HF, Juyal RC, Potocki L, Finucane B, Abuelo DN, Witt DR, Magenis E, Baldini A, Greenberg F, Lupski JR, Patel PI (1996) Quantification by flow cytometry of chromosome-17 deletions in Smith–Magenis syndrome patients. Hum Genet 98:710–718
van Bon BW, Balciuniene J, Fruhman G, Nagamani SC, Broome DL, Cameron E, Martinet D, Roulet E, Jacquemont S, Beckmann JS, Irons M, Potocki L, Lee B, Cheung SW, Patel A, Bellini M, Selicorni A, Ciccone R, Silengo M, Vetro A, Knoers NV, de Leeuw N, Pfundt R, Wolf B, Jira P, Aradhya S, Stankiewicz P, Brunner HG, Zuffardi O, Selleck SB, Lupski JR, de Vries BB (2011) The phenotype of recurrent 10q22q23 deletions and duplications. Eur J Hum Genet 19:400–408. doi:10.1038/ejhg.2010.211
Wang M, Beck CR, English AC, Meng Q, Buhay C, Han Y, Doddapaneni HV, Yu F, Boerwinkle E, Lupski JR, Muzny DM, Gibbs RA (2015) PacBio-LITS: a large-insert targeted sequencing method for characterization of human disease-associated chromosomal structural variations. BMC Genom 16:214. doi:10.1186/s12864-015-1370-2
Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, Shen Y, Yu G, Liu J, Xie H, Gucev ZS, Liu S, Yang N, Al-Kateb H, Chen J, Zhang J, Hauser N, Zhang T, Tasic V, Liu P, Su X, Pan X, Liu C, Wang L, Shen J, Shen J, Chen Y, Zhang T, Zhang J, Choy KW, Wang J, Wang Q, Li S, Zhou W, Guo J, Wang Y, Zhang C, Zhao H, An Y, Zhao Y, Wang J, Liu Z, Zuo Y, Tian Y, Weng X, Sutton VR, Wang H, Ming Y, Kulkarni S, Zhong TP, Giampietro PF, Dunwoodie SL, Cheung SW, Zhang X, Jin L, Lupski JR, Qiu G, Zhang F (2015) TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med 372:341–350. doi:10.1056/NEJMoa1406829
Yan J, Bi W, Lupski JR (2007) Penetrance of craniofacial anomalies in mouse models of Smith–Magenis syndrome is modified by genomic sequence surrounding Rai1: not all null alleles are alike. Am J Hum Genet 80:518–525. doi:10.1086/512043
Yuan B, Harel T, Gu S, Liu P, Burglen L, Chantot-Bastaraud S, Gelowani V, Beck CR, Carvalho CM, Cheung SW, Coe A, Malan V, Munnich A, Magoulas PL, Potocki L, Lupski JR (2015a) Nonrecurrent 17p11.2p12 rearrangement events that result in two concomitant genomic disorders: the PMP22-RAI1 contiguous gene duplication syndrome. Am J Hum Genet 97:691–707. doi:10.1016/j.ajhg.2015.10.003
Yuan B, Liu P, Gupta A, Beck CR, Tejomurtula A, Campbell IM, Gambin T, Simmons AD, Withers MA, Harris RA, Rogers J, Schwartz DC, Lupski JR (2015b) Comparative genomic analyses of the human NPHP1 locus reveal complex genomic architecture and its regional evolution in primates. PLoS Genet 11:e1005686. doi:10.1371/journal.pgen.1005686
Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR (2009) The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat Genet 41:849–853. doi:10.1038/ng.399
Zori RT, Lupski JR, Heju Z, Greenberg F, Killian JM, Gray BA, Driscoll DJ, Patel PI, Zackowski JL (1993) Clinical, cytogenetic, and molecular evidence for an infant with Smith–Magenis syndrome born from a mother having a mosaic 17p11.2p12 deletion. Am J Med Genet 47:504–511. doi:10.1002/ajmg.1320470414
Acknowledgments
We thank the patients and their families for participation. This study was supported in part by the US National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) Grant No. HG006542 to the Baylor-Hopkins Center for Mendelian Genomics (BHCMG); the National Institute of Neurological Disorders and Stroke (NINDS) NS058529, the National Institute of General Medical Sciences (NIGMS) GM106373, the National Institute of Child Health and Development (NICHD) HD024064 Intellectual and Developmental Disabilities Research Center (IDDRC), the National Eye Institute (NEI) EY021163 and EY019861, and the Smith-Magenis Syndrome Research Foundation (SMSRF). T.H. was supported by the Medical Genetics Research Fellowship Program NIH/NIGMS NIH T32 GM007526.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JR.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, has stock options in Lasergen Inc., is a member of the Scientific Advisory Board of Baylor Miraca Genetics Laboratories, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis (CMA) and clinical exome sequencing offered in the Baylor Miraca Genetics Laboratory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, B., Neira, J., Gu, S. et al. Nonrecurrent PMP22-RAI1 contiguous gene deletions arise from replication-based mechanisms and result in Smith–Magenis syndrome with evident peripheral neuropathy. Hum Genet 135, 1161–1174 (2016). https://doi.org/10.1007/s00439-016-1703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-016-1703-5