Abstract
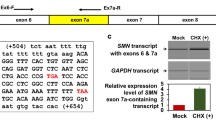
Proximal spinal muscular atrophy (SMA) is a neuromuscular disease caused by low levels of the survival motor neuron (SMN) protein. In humans there are two nearly identical SMN genes, SMN1 and SMN2. The SMN2 gene generates a truncated protein, due to a C to T nucleotide alteration in exon 7, which leads to inefficient RNA splicing of exon 7. This exclusion of SMN exon 7 is central to the onset of the SMA disease. Exon 7 splicing is regulated by a number of exonic and intronic splicing regulatory sequences and the trans-factors that bind them. Here, we identify conserved intronic sequences in the SMN genes. Five regions were examined due to conservation and their proximity to exons 6 through 8. Using mutagenesis two conserved elements located in intron 7 of the SMN genes that affect exon 7 splicing have been identified. Additional analysis of one of these regions showed decreased inclusion of exon 7 in SMN transcripts when deletions or mutations were introduced. Furthermore, multimerization of this conserved region was capable of restoring correct SMN splicing. Together these results describe a novel intronic splicing enhancer sequence located in the final intron of the SMN genes. This discovery provides insight into the splicing of the SMN genes using conserved intonic sequence as a tool to uncover regions of importance in pre-messenger RNA splicing. A better understanding of the way SMN pre-mRNA is spliced can lead to the development of new therapies.






Similar content being viewed by others
References
Baughan TD, Dickson A et al (2009) Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy. Hum Mol Genet 18(9):1600–1611
Black DL (2003) Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 72:291–336
Cartegni L, Krainer AR (2002) Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet 30(4):377–384
Cartegni L, Wang J et al (2003) ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res 31(13):3568–3571
Chen HH, Chang JG et al (2008) The RNA binding protein hnRNP Q modulates the utilization of exon 7 in the survival motor neuron 2 (SMN2) gene. Mol Cell Biol 28(22):6929–6938
Coady TH, Shababi M et al (2007) Restoration of SMN function: delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing. Mol Ther 15(8):1471–1478
Coovert DD, Le TT et al (1997) The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet 6(8):1205–1214
DiDonato CJ, Chen XN et al (1997) Cloning, characterization, and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res 7(4):339–352
DiDonato CJ, Lorson CL et al (2001) Regulation of murine survival motor neuron (Smn) protein levels by modifying Smn exon 7 splicing. Hum Mol Genet 10(23):2727–2736
Hofmann Y, Wirth B (2002) hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum Mol Genet 11(17):2037–2049
Hofmann Y, Lorson CL et al (2000) Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2). Proc Natl Acad Sci USA 97(17):9618–9623
Hua Y, Vickers TA et al (2008) Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet 82(4):834–848
Huang HY, Chien CH et al (2006) RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res 34(Web Server issue):W429–W434
Kashima T, Manley JL (2003) A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat Genet 34(4):460–463
Kashima T, Rao N et al (2007) An intronic element contributes to splicing repression in spinal muscular atrophy. Proc Natl Acad Sci USA 104(9):3426–3431
Lefebvre S, Burglen L et al (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80(1):155–165
Lefebvre S, Burlet P et al (1997) Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 16(3):265–269
Lefebvre S, Burglen L et al (1998) The role of the SMN gene in proximal spinal muscular atrophy. Hum Mol Genet 7(10):1531–1536
Lorson CL, Hahnen E et al (1999) A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 96(11):6307–6311
Miyaso H, Okumura M et al (2003) An intronic splicing enhancer element in survival motor neuron (SMN) pre-mRNA. J Biol Chem 278(18):15825–15831
Monani UR, Lorson CL et al (1999) A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet 8(7):1177–1183
Novelli G, Calza L et al (1997) Expression study of survival motor neuron gene in human fetal tissues. Biochem Mol Med 61(1):102–106
Schrank B, Gotz R et al (1997) Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA 94(18):9920–9925
Singh NK, Singh NN et al (2006) Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol 26(4):1333–1346
Wang W, Furneaux H et al (2000) HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol 20(3):760–769
Young PJ, DiDonato CJ et al (2002) SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2 beta 1. Hum Mol Genet 11(5):577–587
Zhang XH, Chasin LA (2004) Computational definition of sequence motifs governing constitutive exon splicing. Genes Dev 18(11):1241–1250
Acknowledgments
This work was generously supported by The Research Institute at Nationwide Children’s Hospital, and the National Institute of Neurological Disorders and Stroke (NINDS) Grant, 1R21NS054690, to DSC, and the National Institute of General Medical Sciences (NIGMS) Grant, 1F31GM080151-01A1, to JTG. We thank Dr. Brian Kaspar for generously provided the NSC-34 cells and Dr. Ravindra Singh for the Casp3Avr minigene.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2009_733_MOESM1_ESM.eps
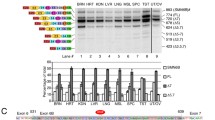
Supplemental Fig. 1 Conservation alignment of the SMN genes. The SMN genes of 17 different vertebrate and invertebrate animals were compared using the 2004 UCSC genome browser. Areas of high conservation are represented by the height of the bar graph at the top of the diagram. The red boxes highlight the areas of conservation that were examined in closer detail. (EPS 916 kb)
439_2009_733_MOESM2_ESM.doc
Supplemental Table 1 Mutagenesis primers used to create the SMN mutations outlined in this paper. Only the sense strand for each primer pair is shown. (DOC 33 kb)
439_2009_733_MOESM3_ESM.doc
Supplemental Table 2 Multispecies alignment of conserved sequence I6-1. The sequences are shown in the reverse complement orientation as depicted using UCSG Genome Browser March 2006 genomic assembly (DOC 29 kb)
439_2009_733_MOESM4_ESM.doc
Supplemental Table 3 Multispecies alignment of conserved sequence I6-2. The sequences are shown in the reverse complement orientation as depicted using UCSG Genome Browser March 2006 genomic assembly. (DOC 40 kb)
439_2009_733_MOESM5_ESM.doc
Supplemental Table 4 Multispecies alignment of conserved sequence I6-3. The sequences are shown in the reverse complement orientation as depicted using UCSG Genome Browser March 2006 genomic assembly. (DOC 29 kb)
439_2009_733_MOESM7_ESM.doc
Supplemental Table 5 Multispecies alignment of conserved sequence I7-1. The sequences are shown in the reverse complement orientation as depicted using UCSG Genome Browser March 2006 genomic assembly. (DOC 29 kb)
439_2009_733_MOESM8_ESM.doc
Supplemental Table 6 Multispecies alignment of conserved sequence I7-2. The sequences are shown in the reverse complement orientation as depicted using UCSG Genome Browser March 2006 genomic assembly. (DOC 31 kb)
Rights and permissions
About this article
Cite this article
Gladman, J.T., Chandler, D.S. Intron 7 conserved sequence elements regulate the splicing of the SMN genes. Hum Genet 126, 833–841 (2009). https://doi.org/10.1007/s00439-009-0733-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0733-7