Abstract
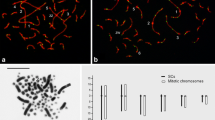
Meiotic recombination is essential for the segregation of homologous chromosomes and the formation of normal haploid gametes. Little is known about patterns of meiotic recombination in human germ cells or the mechanisms that control these patterns. Here, newly developed immunofluorescence techniques, based on the detection of MLH1 (a DNA mismatch repair protein) foci on synaptonemal complexes (SCs) at prophase I of meiosis, were used to examine recombination in human spermatocytes. The mean number of MLH1 foci per cell in all donors was 48.0 with range from 21 to 65. Remarkable variation in the recombination frequency was noted among 11 normal individuals: the mean frequencies of chromosomal recombination foci ranged from a low of 42.5 to a high of 55.0 exchanges. Donor age did not contribute to this variation. There was no correlation between this variation and the frequency of gaps (discontinuities) or splits (unpaired chromosome regions) in the SCs. The mean percentage of cells with gaps was 35% (range: 20% to 58%) and with splits was 7% (range: 0% to 37%). Bivalents without a recombination focus were rare, with a frequency of only 0.3%. Thus, achiasmate chromosomes appear to be rare in human male meiosis.


Similar content being viewed by others
References
Anderson LK, Reeves A, Webb LM, Ashley T (1999) Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 151:1569–1579
Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13:336–342
Barlow AL, Hultén MA (1998) Crossing over analysis at pachytene in man. Eur J Hum Genet 6:350–358
Bascom-Slack C, Ross L, Dawson D (1997) Chiasmata, crossovers, and meiotic chromosome segregation. Adv Genet 35:253–283
Broman K, Murray J, Sheffield V, White R, Weber J (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869
Brooks L (1988) The evolution of recombination rates. In: Michod RE, Levin BR (eds) The evolution of sex: an examination of current ideas. Sinauer, Sunderland, pp 87–105
Egozcue J, Templado C, Vidal F, Navarro J, Morer-Fargas F, Marina S (1983) Meiotic studies in a series of 1100 infertile and sterile males. Hum Genet 65:185–188
Fisher-Lindahl K (1991) His and hers recombinational hotspots. Trends Genet 7:273–276
Fletcher JM (1979) Light microscope analysis of meiotic prophase chromosomes by silver staining. Chromosoma 72:241–248
Froenicke L, Anderson LK, Wienberg J, Ashley T (2002) Male mouse recombination maps for each autosome identified by chromosome painting. Am J Hum Genet 71:1353–1368
Gorlov IP, Zhelezova AI, Gorlova O (1994) Sex differences in chiasma distribution along two marked mouse chromosomes: differences in chiasma distribution as a reason for sex differences in recombination frequency. Genet Res 64:161–166
Hassold T, Sherman S, Hunt P (2000) Counting cross-overs: characterizing meiotic recombination in mammals. Hum Mol Genet 9:2409–2419
Hultén M (1974) Chiasma distribution at diakinesis in the normal human male. Hereditas 76:55–78
Jagiello G, Fang JS (1979) Analyses of diplotene chiasma frequencies in mouse oocytes and spermatocytes in relation to ageing and sexual dimorphism. Cytogenet Cell Genet 23:53–60
Jeffreys AJ, Neumann R (2002) Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat Genet 31:267–271
Jeffreys AJ, Murray J, Neumann R (1998) High-resolution mapping of crossovers in human sperm defines a minisatellite-associated recombination hotspot. Mol Cell 2:267–273
Johannisson R, Schulze W, Holstein AF (2003) Megalospermatocytes in the human testis exhibit asynapsis of chromosomes. Andrologia 35:146–151
Koehler KE, Cherry JP, Lynn A, Hunt PA, Hassold TJ (2002) Genetic control of mammalian meiotic recombination. I. Variation in exchange frequencies among males from inbred mouse strains. Genetics 162: 297–306
Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K (2002) A high-resolution recombination map of the human genome. Nat Genet 31:241–247
Lange R, Krause W, Engel W (1997) Analyses of meiotic chromosomes in testicular biopsies of infertile patients. Hum Reprod 12:2154–2158
Laurie D, Hultén M (1985) Further studies on bivalent chiasma frequency in human males with normal karyotypes. Ann Hum Genet 49: 189–201
Laurie DA, Hulten M, Jones GH (1981) Chiasma frequency and distribution in a sample of human males: chromosomes 1, 2, and 9. Cytogenet Cell Genet 31:153–166
Lawrie NM, Tease C, Hultén MA (1995) Chiasma frequency, distribution and interference maps of mouse autosomes. Chromosoma 104:308–314
Lien S, Szyda J, Schechinger B, Rappold G, Arnheim N (2000) Evidence for heterogeneity in recombination in the human pseudoautosomal region: high resolution analysis by sperm typing and radiation-hybrid mapping. Am J Hum Genet 66:557–566
Luthardt FW, Palmer CG, Yu P (1973) Chiasma and univalent frequencies in aging female mice. Cytogenet Cell Genet 12:68–79
Lynn A, Koehler KE, Judis L, Chan ER, Cherry JP, Schwartz S, Seftel A, Hunt P, Hassold TJ (2002) Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296:2222–2225
Oliver-Bonet M, Liehr T, Nietzel A, Heller A, Starke H, Claussen U, Codina-Pascual M, Pujol A, Abad C, Egozcue J, Navarro J, Benet J (2003) Karyotyping of human synaptonemal complexes by cenM-FISH. Eur J Hum Genet 11:879–883
Park JP, Wojiski SA, Spellman RA, Rhodes CH, Mohandas TK (1998) Human chromosome 9 pericentric homologies: implications for chromosome 9 heteromorphisms. Cytogenet Cell Genet 82:192–194
Reeves R, Crowley M, O’Hara B, Gearhart J (1990) Sex, strain, and species differences affect recombination across an evolutionarily conserved segment of mouse chromosome 16. Genomics 8:141–148
Robinson WP (1996) The extent, mechanism, and consequences of genetic variation, for recombination rate. Am J Hum Genet 59:1175–1183
Roeder GS, Bailis JM (2000) The pachytene checkpoint. Trends Genet 16: 395–403
Rose A, Baillie D (1979) The effect of temperature and parental age on recombination and nondisjunction in C. elegans. Genetics 92:409–418
Shi Q, Spriggs E, Field L, Rademaker A, Ko E, Barclay L, Martin R (2002) Absence of age effect on meiotic recombination between human X and Y chromosomes. Am J Hum Genet 71:254–261
Solari AJ, Ponzio R, Rey Valzacchi G (1991) Synaptonemal complex karyotyping in an oligospermic patient with heterochromatin duplication in chromosome n. 9. Medicina (B Aires) 51:217–221
Speed RM (1977) The effects of ageing on the meiotic chromosomes of male and female mice. Chromosoma 64:241–254
Sugawara S, Mikamo K (1983) Absence of correlation between univalent formation and meiotic nondisjunction in aged female Chinese hamsters. Cytogenet Cell Genet 35:34–40
Sun F, Kozak G, Scott S, Trpkov K, Ko E, Mikhaail-Philips M, Bestor TH, Moens P, Martin RH (2004a) Meiotic defects in a man with non-obstructive azoospermia: case report. Hum Reprod 19:1770–1773
Sun F, Oliver-Bonet M, Liehr T, Starke H, Ko E, Rademaker A, Navarro J, Benet J, Martin RH (2004b) Human male recombination maps for individual chromosomes. Am J Hum Genet 74:521–531
Tanzi RE, Watkins PC, Stewart GD, Wexler NS, Gusella JF, Haines JL (1992) A genetic linkage map of human chromosome 21: analysis of recombination as a function of sex and age. Am J Hum Genet 50:551–558
Tease C, Hartshorne GM, Hultén M (2002) Patterns of meiotic recombination in human fetal oocytes. Am J Hum Genet 70:1469–1479
True JR, Mercer JM, Laurie CC (1996) Differences in crossover frequency and distribution among three sibling species of Drosophila. Genetics 142:507–523
Verma RS, Ramesh KH, Mathews T, Kleyman SM, Conte RA (1996) Centromeric alphoid sequences are breakage prone resulting in pericentromeric inversion heteromorphism of qh region of chromosome 1. Ann Genet 39: 205–208
Vidal F, Templado C, Navarro J, Brusadin S, Marina S, Egozcue J (1982) Meiotic and synaptonemal complex studies in 45 subfertile males. Hum Genet 60:301–304
Vidal F, Navarro J, Templado C, Marina S, Egozcue J (1984) Development and behavior of synaptonemal complexes in human spermatocytes by light and electron microscopy. Hum Genet 68:142–147
Williamson JH, Parker DR, Manchester WG (1970) X-ray-induced recombination in the fourth chromosome of Drosophila melanogaster females. I. Kinetics and brood patterns. Mutat Res 9:287–297
Yu J, Lazzeroni L, Qin J, Huang M, Navidi W, Erlich H, Arnheim N (1996) Individual variation in recombination among human males. Am J Hum Genet 59:1186–1192
Zwick ME, Cutler DJ, Langley CH (1999) Classic Weinstein: tetrad analysis, genetic variation and achiasmate segregation in Drosophila and humans. Genetics 152:1615–1629
Acknowledgements
The authors thank T. Ashley, M. Fritzler, and P. Moens for the generous gift of antibodies. R.H.M. holds the Canada Research Chair in Genetics. This work was funded by the Canadian Institutes of Health Research (CIHR; MA7961). F.S. is the recipient of a CIHR Strategic Training Fellowship in Genetics, Child Development, and Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, F., Trpkov, K., Rademaker, A. et al. Variation in meiotic recombination frequencies among human males. Hum Genet 116, 172–178 (2005). https://doi.org/10.1007/s00439-004-1215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-004-1215-6