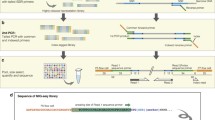
Abstract
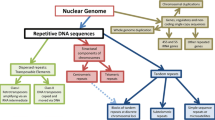
Short, tandemly repeated DNA motifs, termed SSRs (simple sequence repeats) are widely distributed throughout eukaryotic genomes and exhibit a high degree of polymorphism. The availability of size-based methods for genotyping SSRs has made them the markers of choice for genetic linkage studies in all higher eukaryotes. These genotyping methods are not efficiently applicable to mononucleotide repeats (MNRs). Consequently, MNRs, although highly frequent in the genome, have generally been ignored as genetic markers. In contrast to single nucleotide polymorphisms (SNPs), SSRs can be identified in silico once the genomic sequence or segment of interest is available, without requiring any additional information. This makes possible ad-hoc saturation of a target chromosomal region with informative markers. In this context, MNRs appear to have much to offer by increasing the degree of marker saturation that can be obtained. By using the human genome sequence as a model, computational analysis demonstrates that MNRs in the size of 9–15 bp are highly abundant, with an average appearance every 2.9 kb, exceeding di- and tri-nucleotide SSRs frequencies by two- and five-fold, respectively. In order to enable practical, high throughput MNR genotyping, a rapid method was developed, based on sizing of fluorescent-labeled primer extension products. Genotyping of 16 arbitrarily chosen non-coding MNR sites along human chromosome 22 revealed that almost two-thirds (63%) of them were polymorphic, having 2–5 alleles per locus, with 20% of the polymorphic MNRs having more than two alleles. Thus, MNRs have potential for in silico saturation of sequenced eukaryote genomes with informative genetic markers.


Similar content being viewed by others
References
Aitman TJ, Hearne CM, McAleer MA, Todd JA (1991) Mononucleotide repeats are an abundant source of length variants in mouse genomic DNA. Mamm Genome 1:206–210
Atkin NB (2001) Microsatellite instability. Cytogenet Cell Genet 92:177–181
Beckmann JS, Soller M (1990) Toward a unified approach to genetic mapping of eukaryotes based on sequence tagged microsatellite sites. Biotechnology (New York) 8:930–932
Beckmann JS, Weber JL (1992) Survey of human and rat microsatellites. Genomics 12:627–631
Boyer JC, Yamada NA, Roques CN, Hatch SB, Riess K, Farber RA (2002) Sequence dependent instability of mononucleotide microsatellites in cultured mismatch repair proficient and deficient mammalian cells. Hum Mol Genet 11:707–713
Brownstein MJ, Carpten JD, Smith JR (1996) Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques 20:1004–1010
Davies K (1993) Of mice and men (and cows and cats). Nature 361:478
Ellegren H (1993) Abundant (A)n.(T)n mononucleotide repeats in the pig genome: linkage mapping of the porcine APOB, FSA, ALOX12, PEPN and RLN loci. Anim Genet 24:367–372
Field D, Wills C (1998) Abundant microsatellite polymorphism in Saccharomyces cerevisiae, and the different distributions of microsatellites in eight prokaryotes and S. cerevisiae, result from strong mutation pressures and a variety of selective forces. Proc Natl Acad Sci USA 95:1647–1652
Gray IC, Campbell DA, Spurr NK (2000) Single nucleotide polymorphisms as tools in human genetics. Hum Mol Genet 9:2403–2408
Gur-Arie R, Cohen CJ, Eitan Y, Shelef L, Hallerman EM, Kashi Y (2000) Simple sequence repeats in Escherichia coli: abundance, distribution, composition, and polymorphism. Genome Res 10:62–71
Hauge XY, Litt M (1993) A study of the origin of “shadow bands” seen when typing dinucleotide repeat polymorphisms by the PCR. Hum Mol Genet 2:411–415
Kashi Y, King D, Soller M (1997) Simple sequence repeats as a source of quantitative genetic variation. Trends Genet 13:74–78
Katti MV, Ranjekar PK, Gupta VS (2001) Differential distribution of simple sequence repeats in eukaryotic genome sequences. Mol Biol Evol 18:1161–1167
King DG, Soller M, Kashi Y (1997) Evolutionary tuning knobs. Endeavour 21:36–40
Li J, Butler JM, Tan Y, Lin H, Royer S, Ohler L, Shaler TA, Hunter JM, Pollart DJ, Monforte JA, Becker CH (1999) Single nucleotide polymorphism determination using primer extension and time-of-flight mass spectrometry. Electrophoresis 20:1258–1265
Li YC, Korol AB, Fahima T, Beiles A, Nevo E (2002) Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Mol Ecol 11:2453–2465
Maehara Y, Oda S, Sugimachi K (2001) The instability within: problems in current analyses of microsatellite instability. Mutat Res 461:249–263
Metzgar D, Bytof J, Wills C (2000) Selection against frameshift mutations limits microsatellite expansion in coding DNA. Genome Res 10:72–80
Nadir E, Margalit H, Gallily T, Ben Sasson SA (1996) Microsatellite spreading in the human genome: evolutionary mechanisms and structural implications. Proc Natl Acad Sci USA 93:6470–6475
Nickerson DA, Taylor SL, Weiss KM, Clark AG, Hutchinson RG, Stengard J, Salomaa V, Vartiainen E, Boerwinkle E, Sing CF (1998) DNA sequence diversity in a 9.7-kb region of the human lipoprotein lipase gene. Nat Genet 19:233–240
Smeets HJ, Brunner HG, Ropers HH, Wieringa B (1989) Use of variable simple sequence motifs as genetic markers: application to study of myotonic dystrophy. Hum Genet 83:245–251
Tautz D (1989) Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res 17:6463–6471
Taylor JG, Choi EH, Foster CB, Chanock SJ (2001) Using genetic variation to study human disease. Trends Mol Med 7:507–512
Templeton AR, Clark AG, Weiss KM, Nickerson DA, Boerwinkle E, Sing CF (2000) Recombinational and mutational hotspots within the human lipoprotein lipase gene. Am J Hum Genet 66:69–83
Toth G, Gaspari Z, Jurka J (2000) Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res 10:967–981
Weber JL (1990) Informativeness of human (dC-dA)n.(dG-dT)n polymorphisms. Genomics 7:524–530
Webster MT, Smith NG, Ellegren H (2002) Microsatellite evolution inferred from human- chimpanzee genomic sequence alignments. Proc Natl Acad Sci USA 99:8748–8753
Author information
Authors and Affiliations
Corresponding author
Additional information
Helit Cohen and Yael Danin-Poleg contributed equally to this work
Rights and permissions
About this article
Cite this article
Cohen, H., Danin-Poleg, Y., Cohen, C.J. et al. Mono-nucleotide repeats (MNRs): a neglected polymorphism for generating high density genetic maps in silico. Hum Genet 115, 213–220 (2004). https://doi.org/10.1007/s00439-004-1135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-004-1135-5