Abstract
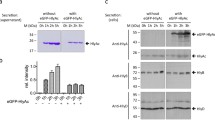
HlyD has a single transmembrane domain (residues 59-80) and a large periplasmic domain, and is essential for the secretion of haemolysin from Escherichia coli. Using an antibody raised against HlyD, the protein was localised to the cell envelope by immunofluorescence and to the cytoplasmic membrane by sucrose gradient analysis. We have examined the stability of this protein in the presence and absence of other putative components of the translocator, HlyB and TolC. HlyD is normally highly stable but in the absence of TolC, the steady-state level of HlyD is greatly reduced and the protein has a half-life at 37° C of 36 min. In the absence of HlyB, HlyD is also unstable and specific degradation products are detected, which co-fractionate with the inner membrane, indicating in this case limited cleavage at specific sites. However, the effect of removing both HlyB and TolC is not additive. On the contrary, in the absence of both HlyB and TolC the half-life of HlyD is approximately 110 min. This result shows that in the presence of HlyB removal of TolC renders HlyD more unstable than it is in the absence of both HlyB and TolC. This suggests that the presence of HlyB induces a structural change in HlyD. In addition, HlyB itself appears to be less stable in the absence of HlyD. These results are consistent with an interaction between HlyD/TolC and HlyB/HlyD. A derivative of HlyD, HlyD22, lacking the 40 N-terminal residues of HlyD assembles into the inner membrane displaying the same stability with and without HlyB as wild type HlyD does. This N-terminal region therefore appears to play no role in stable localisation but is involved in secretion, since HlyD22 is completely secretion defective. Modification of the C-terminus on the other hand completely destabilised the molecule and HlyD was not detectable in the envelope. Secretion of active haemolysin is limited to a brief period during mid to late exponential phase. In contrast, HlyD is apparently synthesised constitutively throughout the growth phase, demonstrating that the production of this component of the translocator is not the limiting factor for growth phase-dependent secretion.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 10 July 1998 / Accepted: 19 October 1998
Rights and permissions
About this article
Cite this article
Pimenta, A., Young, J., Holland, I. et al. Antibody analysis of the localisation, expression and stability of HlyD, the MFP component of the E. coli haemolysin translocator. Mol Gen Genet 261, 122–132 (1999). https://doi.org/10.1007/s004380050949
Issue Date:
DOI: https://doi.org/10.1007/s004380050949