Abstract
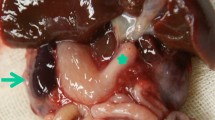
Megacystis-microcolon-hypoperistalsis-syndrome (MMIHS) is a rare and early-onset congenital disease characterized by massive abdominal distension due to a large non-obstructive bladder, a microcolon and decreased or absent intestinal peristalsis. While in most cases inheritance is autosomal dominant and associated with heterozygous variant in ACTG2 gene, an autosomal recessive transmission has also been described including pathogenic bialellic loss-of-function variants in MYH11. We report here a novel family with visceral myopathy related to MYH11 gene, confirmed by whole genome sequencing (WGS). WGS was performed in two siblings with unusual presentation of MMIHS and their two healthy parents. The 38 years-old brother had severe bladder dysfunction and intestinal obstruction, whereas the 30 years-old sister suffered from end-stage kidney disease with neurogenic bladder and recurrent sigmoid volvulus. WGS was completed by retrospective digestive pathological analyses. Compound heterozygous variants of MYH11 gene were identified, associating a deletion of 1.2 Mb encompassing MYH11 inherited from the father and an in-frame variant c.2578_2580del, p.Glu860del inherited from the mother. Pathology analyses of the colon and the rectum revealed structural changes which significance of which is discussed. Cardiac and vascular assessment of the mother was normal. This is the second report of a visceral myopathy corresponding to late-onset form of MMIHS related to compound heterozygosity in MYH11; with complete gene deletion and a hypomorphic allele in trans. The hypomorphic allele harbored by the mother raised the question of the risk of aortic disease in adults. This case shows the interest of WGS in deciphering complex phenotypes, allowing adapted diagnosis and genetic counselling.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Annerén G, Meurling S, Olsen L (1991) Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome (MMIHS), an autosomal recessive disorder: clinical reports and review of the literature. Am J Med Genet 41(2):251–254. https://doi.org/10.1002/ajmg.1320410224
Baek M, DiMaio F, Anishchenko I, Dauparas J, Ovchinnikov S, Lee GR, Wang J et al (2021) Accurate prediction of protein structures and interactions using a three-track neural network. Science 373(6557):871–876. https://doi.org/10.1126/science.abj8754
Billon C, Molin A, Poirsier C, Clemenson A, Dauge C, Grelet M, Sigaudy S et al (2020) Fetal megacystis-microcolon: genetic mutational spectrum and identification of PDCL3 as a novel candidate Gene. Clin Genet 98(3):261–273. https://doi.org/10.1111/cge.13801
Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A (2005) Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet 42(12):881–892. https://doi.org/10.1136/jmg.2004.030171
Dong W, Baldwin C, Choi J, Milunsky JM, Zhang J, Bilguvar K, Lifton RP, Milunsky A (2019) Identification of a dominant MYH11 causal variant in chronic intestinal pseudo-obstruction: Results of whole-exome sequencing. Clin Genet 96(5):473–477. https://doi.org/10.1111/cge.13617. (Epub 2019 Aug 13 PMID: 31389005)
Dulyaninova NG, Bresnick AR (2013) The heavy chain has its day. BioArchitecture 3(4):77–85. https://doi.org/10.4161/bioa.26133
England J, Loughna S (2013) Heavy and light roles: myosin in the morphogenesis of the heart. Cell Mol Life Sci: CMLS 70(7):1221–1239. https://doi.org/10.1007/s00018-012-1131-1
Gauthier J, Bencheikh BOA, Hamdan FF, Harrison SM, Baker LA, Couture F, Thiffault I et al (2015) A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Human Genet EJHG 23(9):1266–1268. https://doi.org/10.1038/ejhg.2014.256
Gosemann J-H, Puri P (2011) Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int 27(10):1041–1046. https://doi.org/10.1007/s00383-011-2954-9
Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, CobanAkdemirs ZH et al (2017) Loss-of-function variants in MYLK cause recessive megacystis microcolon intestinal hypoperistalsis syndrome. Am J Hum Genet 101(1):123–129. https://doi.org/10.1016/j.ajhg.2017.05.011
Halim D, Hofstra RMW, Signorile L, Verdijk RM, van der Werf CS, Sribudiani Y, Brouwer RWW et al (2016) ACTG2 variants impair actin polymerization in sporadic megacystis microcolon intestinal hypoperistalsis syndrome. Hum Mol Genet 25(3):571–583. https://doi.org/10.1093/hmg/ddv497
Halldorsson BV, Eggertsson HP, Moore KHS, Hauswedell H, Eiriksson O, Ulfarsson MO, Palsson G et al (2022) The sequences of 150,119 Genomes in the UK Biobank. Nature 607(7920):732–740. https://doi.org/10.1038/s41586-022-04965-x
Holla OL, Bock G, Busk OL, Isfoss BL (2014) Familial visceral myopathy diagnosed by exome sequencing of a patient with chronic intestinal pseudo-obstruction. Endoscopy 46(6):533–537. https://doi.org/10.1055/s-0034-1365142
Kapur RP (2023) Histopathological, ultrastructural, and immunohistochemical findings in MYH11-variant visceral myopathy. Pediatr Dev Pathol off J Soc Pediatr Pathol Paediatr Pathol Soc 26(1):39–51. https://doi.org/10.1177/10935266221128133
Kapur RP, Goldstein AM, Loeff DS, Myers CT, Paschal CR (2022) Intestinal pathology in patients with pathogenic ACTG2-variant visceral myopathy: 16 patients from 12 families and review of the literature. Pediatr Dev Pathol Offi J Soc Pediatr Pathol Paediatr Pathol Soci 25(6):581–597. https://doi.org/10.1177/10935266221107449
Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581(7809):434–443. https://doi.org/10.1038/s41586-020-2308-7
Kloth K, Renner S, Burmester G, Steinemann D, Pabst B, Lorenz B, Simon R, Kolbe V, Hempel M, Rosenberger G (2019) 16p13 11 Microdeletion uncovers loss-of-function of a MYH11 missense variant in a patient with megacystis-microcolon-intestinal-hypoperistalsis syndrome. Clin Genet 96(1):85–90. https://doi.org/10.1111/cge.13557
Korğalı EÜ, Yavuz A, Şimşek CEÇ, Güney C, Kurtulgan HK, Başer B, Atalar MH, Özer H, Eğilmez HR (2018) Megacystis microcolon intestinal hypoperistalsis syndrome in which a different de novo Actg2 gene mutation was detected: a case report. Fetal Pediatr Pathol 37(2):109–116. https://doi.org/10.1080/15513815.2018.1445149
Lu W, Xiao Y, Huang J, Tao Y, Yan W, Lina Lu, Cao Yi, Cai W (2016) Mutation in actin γ-2 responsible for megacystis microcolon intestinal hypoperistalsis syndrome in 4 Chinese patients. J Pediatr Gastroenterol Nutr 63(6):624–626. https://doi.org/10.1097/MPG.0000000000001204
Matera I, Rusmini M, Guo Y, Lerone M, Li J, Zhang J, Di Duca M et al (2016) Variants of the ACTG2 Gene correlate with degree of severity and presence of megacystis in chronic intestinal pseudo-obstruction. Euro J Human Genet: EJHG 24(8):1211–1215. https://doi.org/10.1038/ejhg.2015.275
Danielle ML, Puri P (2013) Familial megacystis microcolon intestinal hypoperistalsis syndrome: a systematic review. Pediatr Surg Int 29(9):947–951. https://doi.org/10.1007/s00383-013-3357-x
Milewicz DM, Trybus KM, Dong-Chuan Guo H, Sweeney L, Regalado E, Kamm K, Stull JT (2017) Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol 37(1):26–34. https://doi.org/10.1161/ATVBAHA.116.303229
Moreno CA, Sobreira N, Pugh E, Zhang P, Steel G, Torres FR, Cavalcanti DP (2018) Homozygous deletion in MYL9 expands the molecular basis of megacystis–microcolon–intestinal hypoperistalsis syndrome. Eur J Hum Genet 26(5):669–675. https://doi.org/10.1038/s41431-017-0055-5
Prathapan KM, King DE, Raghu VK, Ackerman K, Presel T, Yaworski JA, Ganoza A et al (2021) Megacystis microcolon intestinal hypoperistalsis syndrome: a case series with long-term follow-up and prolonged survival. J Pediatr Gastroenterol Nutr 72(4):e81-85. https://doi.org/10.1097/MPG.0000000000003008
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med off J Am Coll Med Genet 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Sehnal D, Bittrich S, Deshpande M, Svobodová R, Berka K, Bazgier V, Velankar S, Burley SK, Koča J, Rose AS (2021) Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res 49(W1):W431–W437. https://doi.org/10.1093/nar/gkab314
Talisetti A, Longacre T, Pai RK, Kerner J (2009) Diversion colitis in a 19-year-old female with megacystis-microcolon-intestinal hypoperistalsis syndrome. Dig Dis Sci 54(11):2338–2340. https://doi.org/10.1007/s10620-009-0882-5
Thorson W, Diaz-Horta O, Foster J, Spiliopoulos M, Quintero R, Farooq A, Blanton S, Tekin M (2014) De Novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet 133(6):737–742. https://doi.org/10.1007/s00439-013-1406-0
Trebicka J, Biecker E, Gruenhage F, Stolte M, Meier-Ruge WA, Sauerbruch T, Lammert F (2008) Diagnosis of megacystis-microcolon intestinal hypoperistalsis syndrome with aplastic desmosis in adulthood: a case report. Eur J Gastroenterol Hepatol 20(4):353–355. https://doi.org/10.1097/MEG.0b013e3282efa514
Tropeano M, Andrieux J, Collier DA (2014) Clinical utility gene card microdeletion syndrome. Euro J Human Genet 22(5):713–713. https://doi.org/10.1038/ejhg.2013.230
Tuzovic L, Tang S, Miller RS, Rohena L, Shahmirzadi L, Gonzalez K, Li X et al (2015) New insights into the genetics of fetal megacystis: ACTG2 mutations, encoding γ-2 smooth muscle actin in megacystis microcolon intestinal hypoperistalsis syndrome (berdon syndrome). Fetal Diagn Ther 38(4):296–306. https://doi.org/10.1159/000381638
Wang Q, Zhang J, Wang H, Feng Q, Luo F, Xie J (2019) Compound heterozygous variants in MYH11 underlie autosomal recessive megacystis-microcolon-intestinal hypoperistalsis syndrome in a Chinese family. J Hum Genet 64(11):1067–1073. https://doi.org/10.1038/s10038-019-0651-z
Wangler MF, Gonzaga-Jauregui C, Gambin T, Penney S, Moss T, Chopra A, Probst FJ et al (2014) Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet 10(3):e1004258. https://doi.org/10.1371/journal.pgen.1004258
Whittington JR, Poole AT, Dutta EH, Munn MB (2017) A novel mutation in ACTG2 gene in mother with chronic intestinal pseudoobstruction and fetus with megacystis microcolon intestinal hypoperistalsis syndrome. Case Rep Genet 2017:9146507. https://doi.org/10.1155/2017/9146507
Yetman AT, Starr LJ (2018) Newly described recessive MYH11 disorder with clinical overlap of multisystemic smooth muscle dysfunction and megacystis microcolon hypoperistalsis syndromes. Am J Med Genet A 176(4):1011–1014. https://doi.org/10.1002/ajmg.a.38647
Zhu L, Vranckx R, Kien PKV, Lalande A, Boisset N, Mathieu F, Wegman M et al (2006) mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 38(3):343–349. https://doi.org/10.1038/ng1721
Acknowledgements
The authors thank the patients and their parents, as well as all the medical and technical staff of genetics laboratory SeqoiA and Georges Pompidou European Hospital for their cooperation, which made this work possible.
Funding
The authors didn’t receive any specific funding for this workss.
Author information
Authors and Affiliations
Contributions
C Billon, M Hureaux, X Jeunemaitre, GB Piccoli contributed equally to design, analyze of data, redaction and final approval of the manuscript. JM de Saint-Agathe, N Derive contribued to genetics analysis, bioinformatics analysis, modeling of Myh11 protein structure, redaction and final approval of the manuscript. P Bruneval and D Berrebi contributed to pathological analysis, special coloring and immunostaining, redaction and final approval of the manuscript. L Heidet, R Stoeva, contributed to final approval of the manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Ethical approval
Written consent for genetics analysis, publication of pathological images and clinical information was obtained from both index cases and parents, in accordance with French Bioethics Law of 2004. The authors confirm that the study conforms to recognized standards of European Medicines Agency Guidelines for Good Clinical Practice and has been performed in accordance with local ethical approvals.
Additional information
Communicated by Feng Zhang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Billon, C., Piccoli, G.B., de Sainte Agathe, JM. et al. Genome-wide analysis identifies MYH11 compound heterozygous variants leading to visceral myopathy corresponding to late-onset form of megacystis-microcolon-intestinal hypoperistalsis syndrome. Mol Genet Genomics 299, 44 (2024). https://doi.org/10.1007/s00438-024-02136-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00438-024-02136-3