Abstract
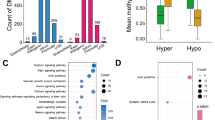
Congenital heart disease (CHD) is a worldwide problem with high morbidity and mortality. Early diagnosis of congenital heart disease is still a challenge in clinical work. In recent years, few studies indicated that placental methylation may be predictors of CHD. More studies are needed to confirm the association between placental methylation and CHD. The aim of this study was to investigate the association between prenatal placental DNA methylation and CHD. Placental tissues were obtained from four fetuses during the second trimester with isolated, non-syndromic congenital heart disease, including three cases with double outlet right ventricle (DORV) and one case with tetralogy of Fallot (TOF), and four unaffected fetuses as controls. The Illumina Infinium Human Methylation 850K BeadChip assay was employed to identify differential methylation sites (DMSs) and differential methylation regions (DMRs). Differential methylation was evaluated by comparing the β-values for individual CpG loci in cases vs. controls. In addition, the function of genes was assessed through KEGG enrichment analysis, Gene Ontology (GO) analysis and KEGG pathway analysis. Compared with the control group, we identified 9625 differential methylation genes on 26,202 DMSs (p < 0.05), of which 6997 were hyper-methylation and 2628 were hypo-methylation. The top 30 terms of GO biological process and KEGG enrichment analysis of DMSs were connected with multiple important pathways of heart development and disease. Ten differentially methylated regions and the genes related to DMRs, such as TLL1, CRABP1, FDFT1, and PCK2, were identified. The deformity caused by the loss of function of these genes is remarkably consistent with the clinical phenotype of our cases. The DNA methylation level of placental tissue is closely associated with fetal congenital heart disease.





Similar content being viewed by others
References
Alanzi T, Alhashem A, Dagriri K, Alzahrani F, Alkuraya FS (2020) A de novo splicing variant supports the candidacy of TLL1 in ASD pathogenesis. Eur J Hum Genet 28(4):525–528
Bahado-Singh R, Vishweswaraiah S, Mishra NK, Guda C, Radhakrishna U (2020) Placental DNA methylation changes in detection of tetralogy of Fallot. Ultrasound Obstet Gynecol 55(6):768–775
Camm EJ, Botting KJ, Sferruzzi-Perri AN (2018) Near to one’s heart: the intimate relationship between the placenta and fetal heart. Front Physiol 9:629
Clark TG, Conway SJ, Scott IC et al (1999) The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development 126(12):2631–2642
Courtney JA, Cnota JF, Jones HN (2018) The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol 9:1045
Daniels TE, Sadovnikoff AI, Ridout KK, Lesseur C, Marsit CJ, Tyrka AR (2020) Associations of maternal diet and placenta leptin methylation. Mol Cell Endocrinol 505:110739
Fantasia I, Andrade W, Syngelaki A, Akolekar R, Nicolaides KH (2019) Impaired placental perfusion and major fetal cardiac defects. Ultrasound Obstet Gynecol 53(1):68–72
Gessert S, Kühl M (2010) The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res 107(2):186–199
Keitges EA, Pasion R, Burnside RD et al (2013) Prenatal diagnosis of two fetuses with deletions of 8p231, critical region for congenital diaphragmatic hernia and heart defects. Am J Med Genet Part A 161(7):1755–1758
Kim S, Cho YH, Lee I et al (2018) Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: a CHECK cohort study. Environ Int 119:398–406
Linask KK (2013) The heart-placenta axis in the first month of pregnancy: induction and prevention of cardiovascular birth defects. J Pregnancy 2013:320413
Ma B, Allard C, Bouchard L et al (2019a) Locus-specific DNA methylation prediction in cord blood and placenta. Epigenetics 14(4):405–420
Ma H, Yu S, Liu X et al (2019b) Lin28a regulates pathological cardiac hypertrophic growth through Pck2-mediated enhancement of anabolic synthesis. Circulation 139(14):1725–1740
Maslen CL (2018) Recent advances in placenta-heart interactions. Front Physiol 9:735
Mohamed IA, El-Badri N, Zaher A (2019) Wnt signaling: the double-edged sword diminishing the potential of stem cell therapy in congenital heart disease. Life Sci 239:116937
Nogues P, Dos Santos E, Jammes H et al (2019) Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin Epigenet 11(1):20
Pierpont ME, Brueckner M, Chung WK et al (2018) Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation 138(21):e653–e711
Radhakrishna U, Albayrak S, Zafra R et al (2019) Placental epigenetics for evaluation of fetal congenital heart defects: ventricular septal defect (VSD). PLoS ONE 14(3):e0200229
Roth GA, Mensah GA, Johnson CO et al (2020) (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: update From the GBD 2019 study. J Am Coll Cardiol 76:2982–3021
Sieron L, Lesiak M, Schisler I, Drzazga Z, Fertala A, Sieron AL (2019) Functional and structural studies of tolloid-like 1 mutants associated with atrial-septal defect 6. Biosci Rep 39(1).
Stachurska E, Loboda A, Niderla-Bielińska J et al (2011) Expression of cellular retinoic acid-binding protein I and II (CRABP I and II) in embryonic mouse hearts treated with retinoic acid. Acta Biochim Pol 58(1):19–29
Su Z, Zou Z, Hay SI et al (2022) Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: an age-period-cohort analysis for the Global Burden of Disease 2019 study. EClinicalMedicine 43:101249
Weiwei C, Runlin G, Lisheng L et al (2016) Outline of the report on cardiovascular diseases in China, 2014. Eur Heart J Suppl 18:2–11
Xiang L, Su Z, Liu Y et al (2018) Effect of family socioeconomic status on the prognosis of complex congenital heart disease in children: an observational cohort study from China. Lancet Child Adolesc Health. 2:430–439
Zhao QM, Liu F, Wu L, Ma XJ, Niu C, Huang GY (2019) Prevalence of congenital heart disease at live birth in China. J Pediatr 204:53–58
Acknowledgements
This work was supported by Beijing Lab for Cardiovascular Precision Medicine, Beijing, China (PXM2020_014226_000017_00377132_FCG) and Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Capital Medical University, Beijing, China (PXM2021_014226_000026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
All participants signed a written consent for inclusion in this study. The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University, gave the final approval for this study.
Additional information
Communicated by Shuhua Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Wu, Y., Sun, H. et al. Association between placental DNA methylation and fetal congenital heart disease. Mol Genet Genomics 298, 243–251 (2023). https://doi.org/10.1007/s00438-022-01944-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-022-01944-9