Abstract
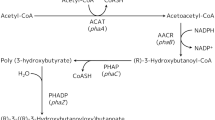
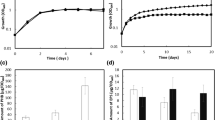
A cluster of genes encoding polyhydroxybutyrate (PHB) depolymerase (phaZ), PHB synthase (phaC), phasin (phaP), and the regulator protein (phaR) was previously identified in Rhodobacter sphaeroides FJ1 (R. sphaeroides FJ1). In this study, we investigated the role of the PhaR protein on the expression of the pha genes. Immunoblot analysis revealed that the expressions of phaP, phaZ and phaR genes in wild-type cells of R. sphaeroides FJ1 are repressed during the active growth phase, with the exception of phaC. A phaR deletion mutant of R. sphaeroides FJ1 was constructed, and the basal level of phaP and phaZ expression in this mutant was markedly increased. Electrophoretic mobility shift assays demonstrated that PhaR binds to the promoter region of phaP as well as those of phaR and phaZ. These results suggest that the PhaR protein is a repressor of phaP, phaR, and phaZ genes in R. sphaeroides FJ1.




Similar content being viewed by others
References
Behrends A, Klingbeil B, Jendrossek D (1996) Poly(3-hydroxybutyrate) depolymerases bind to their substrate by a C-terminal located substrate binding site. FEMS Microbiol Lett 143:191–194
Doudoroff M, Stanier RY (1959) Role of poly-beta-hydroxybutyric acid in the assimilation of organic carbon by bacteria. Nature 183:1440–1442
Hanley SZ, Pappin DJ, Rahman D, White AJ, Elborough KM, Slabas AR (1999) Re-evaluation of the primary structure of Ralstonia eutropha phasin and implications for polyhydroxyalkanoic acid granule binding. FEBS Lett 447:99–105
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Kessler B, Witholt B (2001) Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J Biotechnol 86:97–104
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6:1–8
Liebergesell M, Schmidt B, Steinbuchel A (1992) Isolation and identification of granule-associated proteins relevant for poly(3-hydroxyalkanoic acid) biosynthesis in Chromatium vinosum D. FEMS Microbiol Lett 78:227–232
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Maehara A, Ueda S, Nakano H, Yamane T (1999) Analyses of a polyhydroxyalkanoic acid granule-associated 16-kilodalton protein and its putative regulator in the pha locus of Paracoccus denitrificans. J Bacteriol 181:2914–2921
Maehara A, Doi Y, Nishiyama T, Takagi Y, Ueda S, Nakano H, Yamane T (2001) PhaR, a protein of unknown function conserved among short-chain-length polyhydroxyalkanoic acids producing bacteria, is a DNA-binding protein and represses Paracoccus denitrificans phaP expression in vitro. FEMS Microbiol Lett 200:9–15
Maehara A, Taguchi S, Nishiyama T, Yamane T, Doi Y (2002) A repressor protein, PhaR, regulates polyhydroxyalkanoate (PHA) synthesis via its direct interaction with PHA. J Bacteriol 184:3992–4002
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
Neidle EL, Kaplan S (1993) Expression of the Rhodobacter sphaeroides hemA and hemT genes, encoding two 5-aminolevulinic acid synthase isozymes. J Bacteriol 175:2292–2303
Pieper-Furst U, Madkour MH, Mayer F, Steinbuchel A (1994) Purification and characterization of a 14-kilodalton protein that is bound to the surface of polyhydroxyalkanoic acid granules in Rhodococcus ruber. J Bacteriol 176:4328–4337
Reddy CS, Ghai R, Rashmi Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Saegusa H, Shiraki M, Kanai C, Saito T (2001) Cloning of an intracellular Poly[d(-)-3-Hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J Bacteriol 183:94–100
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schembri MA, Woods AA, Bayly RC, Davies JK (1995) Identification of a 13-kDa protein associated with the polyhydroxyalkanoic acid granules from Acinetobacter spp. FEMS Microbiol Lett 133:277–283
Steinbuchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H (1992) Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev 9:217–230
van der Werf MJ, Zeikus JG (1996) 5-Aminolevulinate production by Escherichia coli containing the Rhodobacter sphaeroides hemA gene. Appl Environ Microbiol 62:3560–3566
Weng SF, Shieh MY, Lai FY, Shao YY, Lin JW, Tseng YH (1996) Construction of a broad-host-range promoter-probing vector and cloning of promoter fragments of Xanthomonas campestris. Biochem Biophys Res Commun 228:386–390
Wieczorek R, Pries A, Steinbuchel A, Mayer F (1995) Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J Bacteriol 177:2425–2435
Wieczorek R, Steinbuchel A, Schmidt B (1996) Occurrence of polyhydroxyalkanoic acid granule-associated proteins related to the Alcaligenes eutrophus H16 GA24 protein in other bacteria. FEMS Microbiol Lett 135:23–30
Yang MK, Wu PI, Yang YC (2000) Identification of a lexA gene in, and construction of a lexA mutant of, Xanthomonas campestris pv. citri. Curr Microbiol 40:233–238
Yang MK, Lin YC, Shen CH (2006) Identification of two gene loci involved in poly-beta-hydroxybutyrate production in Rhodobacter sphaeroides FJ1. J Microbiol Immunol Infect 39:18–27
York GM, Stubbe J, Sinskey AJ (2001) New insight into the role of the PhaP phasin of Ralstonia eutropha in promoting synthesis of polyhydroxybutyrate. J Bacteriol 183:2394–2397
York GM, Stubbe J, Sinskey AJ (2002) The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J Bacteriol 184:59–66
Acknowledgments
We thank Chao-Hung Lee for valuable discussions and critical editing of the manuscript and Shiu-Huey Chou for preparing antiserum to R. sphaeroides Pha proteins. This study was supported by grants (NSC92-2621-Z-030-001 and NSC94-2621-Z-030-001) from the National Science Council, Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klug.
Rights and permissions
About this article
Cite this article
Chou, ME., Chang, WT., Chang, YC. et al. Expression of four pha genes involved in poly-β-hydroxybutyrate production and accumulation in Rhodobacter sphaeroides FJ1. Mol Genet Genomics 282, 97–106 (2009). https://doi.org/10.1007/s00438-009-0448-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-009-0448-4