Abstract
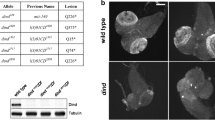
The dominant-negative female-sterile KavarD mutations and their revertant kavarr alleles identify the αTubulin67C gene of Drosophila melanogaster, which codes for the maternally provided α-tubulin4 isoform. The mutations result in the formation of monopolar, collapsed spindles (each with two nearby centrosomes, a tassel of microtubules and overcondensed chromosomes), thus revealing a novel function for α-tubulin4 in spindle maintenance and elongation. Molecular features of the two KavarD alleles and a kavarnull allele are described and models for their actions are discussed.



Similar content being viewed by others
References
Adams MD et al (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195
Baker J, Theurkauf WE, Schubiger G (1993) Dynamic changes in microtubule configuration correlate with nuclear migration in the preblastoderm Drosophila embryo. J Cell Biol 122:113–121
Cytrynbaum EN, Scholey JM, Mogilner A (2003) A force balance model of early spindle pole separation in Drosophila embryos. Biophys J 84:757–769
Deák P et al (1997) P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: Correlation of physical and cytogenetic maps in chromosomal region 86E–87F. Genetics 147:1697–1722
Erdélyi M, Szabad J (1989) Isolation and characterization of dominant female sterile mutations of Drosophila melanogaster. I. Mutations on the third chromosome. Genetics 122:111–127
Foe VA, Odell GM, Edgar BA (1993) Mitosis and morphogenesis in the Drosophila embryo. In: Bate M, Arias AM (eds) The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, New York, pp 149–300
Heck MM, Pereira A, Pesavento P, Yannoni Y, Spradling AC, Goldstein LS (1993) The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J Cell Biol 123:665–679
Heuer JG, Li K, Kaufman TC (1995) The Drosophila homeotic target gene centrosomin (cnn) encodes a novel centrosomal protein with leucine zippers and maps to a genomic region required for midgut morphogenesis. Development 121:3861–3876
Kalfayan L, Wensink PC (1982) Developmental regulation of Drosophila alpha-tubulin genes. Cell 29:91–98
Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH (2002) Microtubule structure at 8 Å resolution. Structure (Camb) 10:1317–1328
Lindsley DL, Zimm GG (1992) The Genome of Drosophila melanogaster. Academic Press, San Diego
Löwe J, Li H, Downing KH, Nogales E (2001) Refined structure of alpha beta-tubulin at 3.5 Å resolution. J Mol Biol 313:1045–1057
Máthé E, Boros I, Jósvay K, Li K, Puro J, Kaufman TC, Szabad J (1998) The Tomaj mutant alleles of alpha Tubulin67C reveal a requirement for the encoded maternal specific tubulin isoform in the sperm aster, the cleavage spindle apparatus and neurogenesis during embryonic development in Drosophila. J Cell Sci 111:887–896
Matthews KA, Miller DF, Kaufman TC (1989) Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev Biol 132:45–61
Matthews KA, Rees D, Kaufman TC (1993) A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development 117:977–991
Matthies HJ, Messina LG, Namba R, Greer KJ, Walker MY, Hawley RS (1999) Mutations in the alpha-tubulin 67C gene specifically impair achiasmate segregation in Drosophila melanogaster. J Cell Biol 147:1137–1144
Menendez M, Rivas G, Diaz JF, Andreu JM (1998) Control of the structural stability of the tubulin dimer by one high affinity bound magnesium ion at nucleotide N-site. J Biol Chem 273:167–176
Mischke D, Pardue ML (1982) Organization and expression of alpha-tubulin genes in Drosophila melanogaster. one member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic development. J Mol Biol 156:449–466
Mizuno N, Toba S, Edamatsu M, Watai-Nishii J, Hirokawa N, Toyoshima YY, Kikkawa M (2004) Dynein and kinesin share an overlapping microtubule-binding site. EMBO J 23:2459–2467
Nogales E (1999) A structural view of microtubule dynamics. Cell Mol Life Sci 56:133–142
Pokrywka NJ, Meng L, Debiec K, Stephenson EC (2004) Identification of hypomorphic and null alleles of swallow via molecular and phenotypic analyses. Dev Genes Evol 214:185–192
Robinson JT, Wojcik EJ, Sanders MA, McGrail M, Hays TS (1999) Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J Cell Biol 146:597–608
Rogers GC, Rogers SL, Schwimmer TA, Ems-McClung SC, Walczak CE, Vale RD, Scholey JM, Sharp DJ (2004) Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427:364–370
Scholey JM, Brust-Mascher I, Mogilner A (2003) Cell division. Nature 422:746–752
Sharp DJ, Yu KR, Sisson JC, Sullivan W, Scholey JM (1999) Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat Cell Biol 1:51–54
Sharp DJ, Rogers GC, Scholey JM (2000a) Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nat Cell Biol 2:922–930
Sharp DJ, Rogers GC, Scholey JM (2000b) Microtubule motors in mitosis. Nature 407:41–47
Sharp DJ, Brown HM, Kwon M, Rogers GC, Holland G, Scholey JM (2000c) Functional coordination of three mitotic motors in Drosophila embryos. Mol Biol Cell 11:241–253
Theurkauf WE (1992) Behavior of structurally divergent alpha-tubulin isotypes during Drosophila embryogenesis: evidence for post-translational regulation of isotype abundance. Dev Biol 154:205–217
Theurkauf WE, Baum H, Bo J, Wensink PC (1986) Tissue-specific and constitutive alpha-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc Natl Acad Sci USA 83:8477–8481
Wieschaus E, Nüsslein-Volhard C (1986) Looking at embryos. In: Roberts DB (ed) Drosophila: a practical approach. IRL Press, Oxford, pp 199–228
Acknowledgements
We thank Thomas C. Kaufman for the generous gift of the CNN antibody, Steffi Kuhfittig for Df(3L)55, Kathy Matthews for the αTub67C− alleles, Endre Máthé for Df(3L)21mrX1 and Thomas Rudolph for the Drosophila genomic λ library. Support for our work came from the following sources: the Hungarian Academy of Sciences, Maternal-Effect and Embryogenesis Research Group, the FKFP (Grant No. 1348), and the Graduate Student Program of the University of Szeged
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Reuter
Rights and permissions
About this article
Cite this article
Venkei, Z., Szabad, J. The KavarD dominant female-sterile mutations of Drosophila reveal a role for the maternally provided α-tubulin4 isoform in cleavage spindle maintenance and elongation. Mol Genet Genomics 273, 283–289 (2005). https://doi.org/10.1007/s00438-005-1109-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-1109-x