Abstract
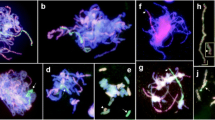
To elucidate the genetic system that establishes homologous chromosome pairing in monocot plants, we have isolated an asynaptic mutant of rice, designated pair2 (homologous p airing a berration i n r ice meiosis 2), in which 24 completely unpaired univalents are observed at pachytene and diakinesis. The mutation was caused by an insertion of the retrotransposon Tos17, as demonstrated by complementation of the mutation by transformation with the corresponding wild-type gene. The gene in which the element was inserted is orthologous to the ASY1 gene of Arabidopsis thaliana and the HOP1 gene of Saccharomyces cerevisiae. Mature PAIR2 mRNA and several splicing variants were found to be highly expressed in wild-type reproductive tissues, and lower expression was also detected in vegetative tissues. In situ hybridization and BrdU incorporation experiments revealed that PAIR2 expression is specifically enhanced in male and female meiocytes, but not in those at pre-meiotic S phase or in the pollen maturation stages. The results obtained in this study suggest that the PAIR2 gene is essential for homologous chromosome pairing in meiosis, as in the case of the genes ASY1 and HOP1. The study also suggested the possibility that a highly homologous copy of the PAIR2 gene located on a different chromosome is in fact a pseudogene.







Similar content being viewed by others
References
Ahn S, Anderson JA, Sorrells ME, Tanksley SD (1993) Homoeologous relationships of rice, wheat and maize chromosomes. Mol Gen Genet 241:483–490
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Aravind L, Koonin EV (1998) The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem Sci 23:284–286
Armstrong SJ, Caryl AP, Jones GH, Franklin FC (2002) ASY1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J Cell Sci 115:3645–3655
Bailis JM, Roeder GS (2000) Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell 101:211–221
Bhatt AM, Canales C, Dickinson HG (2001) Plant meiosis: the means to 1 N. Trends Plant Sci 6:114–121
Caryl AP, Armstrong SJ, Jones GH, Franklin FC (2000) A homologue of the yeast HOP1 gene is inactivated in the Arabidopsis meiotic mutant asy1. Chromosoma 109:62–71
Caryl AP, Jones GH, Franklin FC (2003) Dissecting plant meiosis using Arabidopsis thaliana mutants. J Exp Bot 54:25–38
Fuse T, Sasaki T, Yano M (2001) Ti-plasmid vectors useful for functional analysis of rice genes. Plant Biotechnol 18:219–222
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice ( Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hirochika H, Sugimoto K, Otsuki Y, Tsugawa H, Kanda M (1996) Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci USA 93:7783–7788
Hollingsworth NM, Ponte L (1997) Genetic interactions between HOP1, RED1 and MEK1 suggest that MEK1 regulates assembly of axial element components during meiosis in the yeast Saccharomyces cerevisiae. Genetics 147:33–42
Hollingsworth NM, Goetsch L, Byers B (1990) The HOP1 gene encodes a meiosis-specific component of yeast chromosomes. Cell 61:73–84
Kitada K, Omura T (1983) Genetic control of meiosis in rice Oryza sativa L. II. Cytogenetical analyses of desynaptic mutants. Jpn J Genet 58:567–577
Kitada K, Kurata N, Satoh H, Omura T (1983) Genetic control of meiosis in rice, Oryza sativa L. I. Classification of meiotic mutants induced by MNU and their cytogenetical characteristics. Jpn J Genet 58:231–240
Kouchi H, Hata S (1993) Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet 238:106–119
Kurata N, Omura T, Iwata N (1981) Studies on centromere, chromomere and nucleolus in pachytene nuclei of rice, Oryza sativa , microsporocytes. Cytologia 46:791–800
Li HW, Pao WK, Li CH (1945) Desynapsis in the common wheat. Am J Bot 32:92–101
Martinez-Perez E, Shaw P, Reader S, Aragon-Alcaide L, Miller T, Moore G (1999) Homologous chromosome pairing in wheat. J Cell Sci 112:1761–1769
Moore G, Devos KM, Wang Z, Gale MD (1995) Cereal genome evolution: grasses, line up and form a circle. Curr Biol 5:737–739
Nonomura KI, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N (2003) The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell 15:1728–1739
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Suzuki T, Kawano S, Sakai A, Fujie M, Kuroiwa H, Nakamura H, Kuroiwa T (1992) Preferential mitochondrial and plastid DNA synthesis before multiple cell divisions in Nicotiana tabacum. J Cell Sci 103:831–837
Von Wettstein D, Rasmussen SW, Holm PB (1984) The synaptonemal complex in genetic segregation. Annu Rev Genet 18:331–413
Watanabe Y, Yokobayashi S, Yamamoto M, Nurse P (2001) Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature 409:359–363
Woltering D, Baumgartner B, Bagchi S, Larkin B, Loidl J, de los Santos T, Hollingsworth NM (2000) Meiotic segregation, synapsis, and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol Cell Biol 20:6646–6658
Yamazaki M, Tsugawa H, Miyao A, Yano M, Wu J, Yamamoto S, Matsumoto T, Sasaki T, Hirochika H (2001) The rice retrotransposon Tos17 prefers low-copy-number sequences as integration targets. Mol Genet Genomics 265:336–344
Zetka MC, Kawasaki I, Strome S, Muller F (1999) Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev 13:2258–2270
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33:603–754
Acknowledgements
We would like to thank A. Morohoshi for technical assistance. We also thank Dr. M. Yano (N.I.A.S., Tsukuba, Japan) for providing vectors for rice transformation. This research was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences, Japan, and in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project MP-2112).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Jürgens
Rights and permissions
About this article
Cite this article
Nonomura, KI., Nakano, M., Murata, K. et al. An insertional mutation in the rice PAIR2 gene, the ortholog of Arabidopsis ASY1, results in a defect in homologous chromosome pairing during meiosis. Mol Genet Genomics 271, 121–129 (2004). https://doi.org/10.1007/s00438-003-0934-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0934-z