Abstract
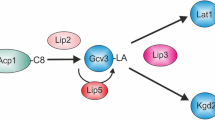
While de novo fatty acid synthesis uses acetyl-CoA, fatty acid elongation uses longer-chain acyl-CoAs as primers. Several mutations that interfere with fatty acid elongation in yeast have already been described, suggesting that there may be different elongases for medium- and long-chain acyl-CoA primers. In the present study, an experimental approach is described that allows differential characterization of the various yeast elongases in vitro. Based on their characteristic primer specificities and product patterns, at least three different yeast elongases are defined. Elongase I extends C12–C16 fatty acyl-CoAs to C16–C18 fatty acids. Elongase II elongates palmitoyl-CoA and stearoyl-CoA up to C22 fatty acids, and elongase III synthesizes 20–26-carbon fatty acids from C18-CoA primers. Elongases I, II and III are specifically inactivated in, respectively, elo1, elo2 and elo3 mutants. Elongases II and III share the same 3-ketoacyl reductase, which is encoded by the YBR159w gene. Inactivation of YBR159w inhibits in vitro fatty acid elongation after the first condensation reaction. Although in vitro elongase activity is absent, the mutant nevertheless contains 10–30% of normal VLCFA levels. On the basis of this finding, an additional elongating activity is inferred to be present in vivo. ybr159Δ cells show synthetic lethality in the presence of cerulenin, which inactivates fatty acid synthase. An involvement of FAS in VLCFA synthesis may account for these findings, but remains to be demonstrated directly. Alternatively, a vital role for C18 and C20 hydroxyacids, which are dramatically overproduced in ybr159Δ cells, may be postulated.







Similar content being viewed by others
Notes
Abbreviations: FAS, fatty acid synthase; CER, cerulenin; PMSF, phenylmethane sulfonyl fluoride; VLCFA, very-long-chain fatty acid; YEP, yeast extract, peptone, sucrose; YEPFA, fatty acid containing YEP; n:0, saturated fatty acid containing n carbon atoms; Cn, fatty acid containing n carbon atoms; n-OH, hydroxylated fatty acid with n carbon atoms
References
Beaudoin F, Michaelson LV, Hey SJ, Lewis MJ, Shewy PR, Sayanova O, Napier JA (2000) Heterologous reconstitution in yeast of the polyunsaturated fatty acid biosynthetic pathway. Proc Natl Acad Sci USA 97:6421–6426
Beaudoin F, Gable K, Sayanova O, Dunn T, Napier J (2002) A Saccharomyces cerevisiae gene required for heterologous fatty acid elongase activity encodes a microsomal β-keto-reductase. J Biol Chem 227:11481–11488
Bloch K, Vance D (1977) Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem 46:263–298
Brody S, Oh C, Hoja U, Schweizer E (1997) Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett 408:217–220
Cassagne C, Darriet D, Bourre JM (1978) Biosynthesis of very long chain fatty acids by the sciatic nerve of the rabbit. FEBS Lett 90:336–340
Dittrich F, Zajonc D, Hühne K, Hoja U, Ekici A, Greiner E, Klein H, Hofmann J, Bessoule JJ, Sperling P, Schweizer E (1998) Fatty acid elongation in yeast. Biochemical characteristics of the enzyme system and isolation of elongation-defective mutants. Eur J Biochem 252:477–485
Douglas WJ, Lim E, Keller J, Plooy I, Ralston E, Dooner HK (1995) Directed tagging of the Arabidopsis F ATTY ACID ELONGATION 1 ( FAE1) gene with the maize transposon Activator. Plant Cell 7:309–319
Gari E, Piedrafita L, Aldea M, Herrero E (1997) A set of vectors with a tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast 13:837–848
Han G, Gable K, Kohlwein S, Beaudoin F, Napier J, Dunn TM (2002) The Saccharomyces cerevisiae YBR159w gene encodes the 3-ketoreductase of the microsomal fatty acid elongase. J Biol Chem 277:35440–35449
Kohlwein S, Eder S, Oh CS, Martin CE, Gable K, Bacikova, D, Dunn T (2001) Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear-vacuolar interface in Saccharomyces cerevisiae. Mol Cell Biol 21:109–125
Kresze GB, Steber L, Oesterhelt D, Lynen F (1977) Reaction of yeast fatty acid synthetase with iodoacetamide. 3. Malonyl-Coenzyme A decarboxylase as product of the reaction of fatty acid synthase with iodoacetamide. Eur J Biochem 79:191–199
Lynen F (1969) Yeast fatty acid synthase. Methods Enzymol 14:17–33
Millar AA, Kunst L (1997) Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J 12:121–131
Milton AM, Axelrod AE, Hofmann K (1950) The synthesis of higher aliphatic β-keto acids. J Am Chem Soc 72:1231–1232
Oh CS, Toke DA, Mandala S, Martin CE (1997) ELO2 and ELO3, homologs of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem 272:17376–17384
Omura S (1976) The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev 40:681–697
Schneiter R, Kohlwein S (1997) Organelle structure, function, and inheritance in yeast: a role for fatty acid synthesis? Cell 88:431–434
Schneiter R, Guerra CE, Lampl M, Gogg G, Kohlwein S, Klein HL (1999) The Saccharomyces cerevisiae hyperrecombination mutant hpr1Δ is synthetically lethal with two conditional alleles of the acetyl coenzyme A carboxylase gene and causes a defect in nuclear export of polyadenylated RNA. Mol Cell Biol 19:3415–3422
Todd J, Post-Beittenmiller D, Jaworski JG (1999) KCS1encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Toke DA, Martin CE (1996) Isolation and characterization of a gene affecting fatty acid elongation in Saccharomyces cerevisiae. J Biol Chem 271:18413–18422
Wach A, Brachat A, Alberti-Segni C, Rebischung C, Philippsen P (1997) Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast 13:1065–1075
Welch JW, Burlingame AL (1973) Very long chain fatty acids in yeast. J Bacteriol 115:464–466
Yalpani M, Willecke K, Lynen F (1969) Triacetic acid lactone, a derailment product of fatty acid biosynthesis. Eur J Biochem 8:495–502
Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11: 2187–2201
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft and by the Fonds der Chemischen Industrie. The work has been carried out in compliance with the current laws governing genetic experimentation in Germany
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. P. Hollenberg
Rights and permissions
About this article
Cite this article
Rössler, H., Rieck, C., Delong, T. et al. Functional differentiation and selective inactivation of multiple Saccharomyces cerevisiae genes involved in very-long-chain fatty acid synthesis. Mol Gen Genomics 269, 290–298 (2003). https://doi.org/10.1007/s00438-003-0836-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0836-0