Abstract
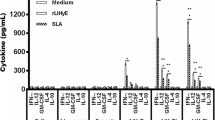
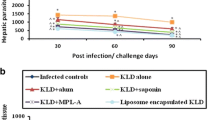
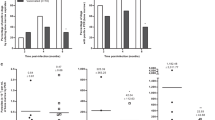
Tegumentary leishmaniasis (TL) is the main clinical manifestation of leishmaniasis, and it can cause the infected hosts to self-healing cutaneous lesions until mutilating scars in mucosal membranes, particularly in the nose and throat. The treatment against disease presents problems, and the diagnosis is hampered by variable sensitivity and/or specificity of the tests. In this context, the development of prophylactic vaccines could be considered as a strategy to control the disease. Previously, we showed that the recombinant LiHyp1 protein plus adjuvant protected mice from infection with Leishmania infantum, which causes visceral leishmaniasis. In the present study, we tested whether rLiHyp1 could induce protection against infection with L. amazonensis, a parasite species able to cause TL. We immunized BALB/c mice with rLiHyp1 plus saponin (rLiHyp1/S) or incorporated in micelles (rLiHyp1/M) as adjuvants and performed parasitological and immunological evaluations before and after infection. Results showed that after in vitro stimulation from spleen cell cultures using rLiHyp1 or a Leishmania antigenic extract (SLA), rLiHyp1/S and rLiHyp1/M groups developed a Th1-type immune response, which was characterized by high levels of IFN-γ, IL-2, TNF-α and IL-12 cytokines, nitrite, and IgG2a isotype antibodies when compared to values found in the control (saline, saponin, micelles alone) groups, which showed higher levels of anti-SLA IL-4, IL-10, and IgG1 antibodies before and after challenge. In addition, mice receiving rLiHyp1/S or rLiHyp1/M presented significant reductions in the lesion average diameter and parasite load in the infected tissue and internal organs. Blood samples were collected from healthy subjects and TL patients to obtain PBMC cultures, which were in vitro stimulated with rLiHyp1 or SLA, and results showed higher lymphoproliferation and IFN-γ production after stimulus using rLiHyp1, as compared to values found using SLA. These results suggest that rLiHyp1 plus adjuvant was protective against experimental TL and could also be considered for future studies as a vaccine candidate against human disease.








Similar content being viewed by others
Data availability
All data are included in the manuscript.
References
Almeida GG, Coura FM, Barbieri JM, Moura ACJ, Paes-Leme FO, Costa-Val AP (2021) FML/QuilA-Vaccinated Dogs Naturally Infected with Leishmania infantum: Serum Cytokines, Clinicopathological Profile, and Parasitological Parameters. Biomed Res Int 2021:3192960. https://doi.org/10.1155/2021/3192960
Asad M, Sabur A, Kamran M, Shadab M, Das S, Ali N (2021) Effector functions of Th17 cells are regulated by IL-35 and TGF-β in visceral leishmaniasis. FASEB J 35:e21755
Asad M, Sabur A, Shadab M, Das S, Kamran M, Didwania N, Ali N (2019) EB1-3 chain of IL-35 along with TGF-β synergistically regulate anti-leishmanial immunity. Front Immunol 10:616
Azeredo-Coutinho RB, Conceição-Silva F, Schubach A, Cupolillo E, Quintella LP, Madeira MF, Pacheco RS, Valete-Rosalino CM, Mendonça SC (2007) First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans R Soc Trop Med Hyg 101:735–737
Banerjee A, Bhattacharya P, Joshi AB, Ismail N, Dey R, Nakhasi HL (2016) Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol 309:37–41
Barral A, Pedral-Sampaio D, Grimaldi Júnior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, Almeida R, Badaro R, Barral-Netto M, Carvalho EM, Johnson WD Jr (1991) Leishmaniasis in Bahia, Brazil: evidence that Leishmaniaamazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg 44(5):536–46. https://doi.org/10.4269/ajtmh.1991.44.536
Bayih AG, Daifalla NS, Gedamu L (2017) Immune response and protective efficacy of a heterologous DNA-protein immunization with Leishmania superoxide dismutase B1. Biomed Res Int 2017:2145386
Bodratti AM, Alexandridis P (2018) Amphiphilic block copolymers in drug delivery: advances in formulation structure and performance. Expert Opin Drug Deliv 15:1085–1104
Brito RCF, Ruiz JC, Cardoso JMO, Ostolin T, Reis LES, Mathias FAS, Aguiar-Soares RDO, Roatt BM, Corrêa-Oliveira R, Resende DM, Reis AB (2020) Chimeric vaccines designed by immunoinformatics-activated polyfunctional and memory T cells that trigger protection against experimental visceral leishmaniasis. Vaccines (Basel) 8:252
Coelho EAF, Tavares CA, Carvalho FA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994
Coelho VTS, Oliveira JS, Valadares DG, Chávez-Fumagalli MA, Duarte MC, Lage PS, Soto M, Santoro MM, Tavares CA, Fernandes AP, Coelho EA (2012) Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis 6:1–10
Coeshott CM, Smithson SL, Verderber E, Samaniego A, Blonder JM, Rosenthal GJ (2004) Westerink MA. Pluronic F127-based systemic vaccine delivery systems. Vaccine 22:2396–2405
Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA (2010) IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11:1093–1101
Duarte MC, Lage LM, Lage DP, Martins VT, Carvalho AM, Roatt BM, Menezes-Souza D, Tavares CA, Alves RJ, Barichello JM, Coelho EA (2016) Treatment of murine visceral leishmaniasis using an 8-hydroxyquinoline-containing polymeric micelle system. Parasitol Int 65:728–736
Dubie T, Mohammed Y (2020) Review on the role of host immune response in protection and immunopathogenesis during cutaneous leishmaniasis infection. J Immunol Res 2020:2496713
Dutra WO, Barbosa DF, Souza PEA, Morgan D, Poetker S, Guimarães LH, Bacelar O, Gollob KJ, Carvalho EM (2019) A Th2-type response is associated with exuberant lesions in pregnant women infected with Leishmania braziliensis. J Infect Dis 219:480–488
Freitas CS, Lage DP, Machado AS, Vale DL, Martins VT, Cardoso JMO, Oliveira-da-Silva JA, Reis TAR, Tavares GSV, Ramos FF, Ludolf F, Pereira IAG, Bandeira RS, Fujiwara RT, Bueno LL, Roatt BM, Chávez-Fumagalli MA, Coelho EAF (2023) Exploring drug repositioning for leishmaniasis treatment: ivermectin plus polymeric micelles induce immunological response and protection against tegumentary leishmaniasis. Cytokine 164:156143
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386–401
Giunchetti RC, Corrêa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, Roatt BM, de Oliveira Aguiar-Soares RD, de Souza JV, das Dores Moreira N, Malaquias LC, Castro LL M e, de Lana M, Reis AB (2007) Immunogenicity of a killed Leishmania vaccine with saponin adjuvant in dogs. Vaccine 25:7674–7686
Grimaldi G Jr, Tesh RB (1993) Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev 6:230–250
Hojatizade M, Soleymani M, Tafaghodi M, Badiee A, Chavoshian O, Jaafari MR (2018) Chitosan nanoparticles loaded with whole and soluble Leishmania antigens, and evaluation of their immunogenecity in a mouse model of leishmaniasis. Iran J Immunol 15:281–293
Jesus-Pereira NC, Régis WC, Costa LE, Oliveira JS, Silva AG, Martins VT, Duarte MC, Souza JR, Lage PS, Schneider MS, Melo MN, Soto M, Soares SA, Tavares CA, Chávez-Fumagalli MA, Coelho EA (2015) Evaluation of adjuvant activity of fractions derived from Agaricus blazei, when in association with the recombinant LiHyp1 protein, to protect against visceral leishmaniasis. Exp Parasitol 153:180–190
Koger-Pease C, Perera DJ, Ndao M (2023) Recent advances in the development of adenovirus-vectored vaccines for parasitic infections. Pharmaceuticals (Basel) 16:334
Kumari S, Samant M, Misra P, Khare P, Sisodia B, Shasany AK, Dube A (2008) Th1-stimulatory polyproteins of soluble Leishmania donovani promastigotes ranging from 89.9 to 97.1 kDa offers long-lasting protection against experimental visceral leishmaniasis. Vaccine 26:5700–5711
Lage DP, Machado AS, Freitas CS, Vale DL, Linhares FP, Cardoso JMO, Oliveira-da-Silva JA, Ramos FF, Pereira IAG, Ludolf F, Tavares GSV, Bandeira RS, Oliveira JS, Menezes-Souza D, Duarte MC, Galdino AS, Christodoulides M, Chávez-Fumagalli MA, Roatt BM et al (2023) Recombinant endonuclease III protein from Leishmania infantum associated with Th1-type adjuvants is immunogenic and induces protection against visceral leishmaniasis. Mol Immunol 155:79–90
Lage DP, Machado AS, Vale DL, Freitas CS, Linhares FP, Cardoso JMO, Pereira IAG, Ramos FF, Tavares GSV, Ludolf F, Oliveira-da-Silva JA, Bandeira RS, Silva AM, Simões LC, Reis TAR, Oliveira JS, Christodoulides M, Chávez-Fumagalli MA, Roatt BM et al (2022) Recombinant guanosine-5’-triphosphate (GTP)-binding protein associated with Poloxamer 407-based polymeric micelles protects against Leishmania infantum infection. Cytokine 153:155865
Lage DP, Ribeiro PAF, Dias DS, Mendonça DVC, Ramos FF, Carvalho LM, Steiner BT, Tavares GSV, Martins VT, Machado AS, Oliveira-da-Silva JA, Santos TTO, Freitas CS, Oliveira JS, Roatt BM, Machado-de-Ávila RA, Humbert MV, Christodoulides M, Coelho EAF (2020) Liposomal formulation of ChimeraT, a multiple T-cell epitope-containing recombinant protein, is a candidate vaccine for human visceral leishmaniasis. Vaccines (Basel) 8:289
Machado AS, Lage DP, Vale DL, Freitas CS, Linhares FP, Cardoso JMO, Oliveira-da-Silva JA, Pereira IAG, Ramos FF, Tavares GSV, Ludolf F, Bandeira RS, Maia LGN, Menezes-Souza D, Duarte MC, Chávez-Fumagalli MA, Roatt BM, Christodoulides M, Martins VT, Coelho EAF (2022) Leishmania LiHyC protein is immunogenic and induces protection against visceral leishmaniasis. Parasite Immunol 44:e12921
Martins VT, Chávez-Fumagalli MA, Costa LE, Canavaci AM, Martins AM, Lage PS, Lage DP, Duarte MC, Valadares DG, Magalhães RD, Ribeiro TG, Nagem RA, DaRocha WD, Régis WC, Soto M, Coelho EA, Fernandes AP, Tavares CA (2013) Antigenicity and protective efficacy of a Leishmania amastigote-specific protein, member of the super-oxygenase family, against visceral leishmaniasis. PLoS Negl Trop Dis 7:e2148
Mirzaei A, Maleki M, Masoumi E, Maspi N (2021) A historical review of the role of cytokines involved in leishmaniasis. Cytokine 145:155297
Olekhnovitch R, Bousso P (2015) Induction, propagation, and activity of host nitric oxide: lessons from Leishmania infection. Trends Parasitol 31:653–664
Oliveira JP, Fernandes F, Cruz AK, Trombela V, Monteiro E, Camargo AA, Barral A, Oliveira CI (2007) Genetic diversity of Leishmania amazonensis strains isolated in northeastern Brazil as revealed by DNA sequencing, PCR-based analyses and molecular karyotyping. Kinetoplastid Biol Dis 6:5
Oliveira-da-Silva JA, Machado AS, Tavares GSV, Ramos FF, Lage DP, Ludolf F, Steiner BT, Reis TAR, Santos TTO, Costa LE, Bandeira RS, Martins VT, Galvani NC, Chaves AT, Oliveira JS, Chávez-Fumagalli MA, Tupinambás U, de Magalhães-Soares DF, Silveira JAG et al (2020) Biotechnological applications from a Leishmania amastigote-specific hypothetical protein in the canine and human visceral leishmaniasis. Microb Pathog 147:104283
Palatnik-de-Sousa CB (2008) Vaccines for leishmaniasis in the fore coming 25 years. Vaccine 26:1709–1724
Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva LA, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC, Correa-Oliveira R (2005) Immune response in human visceral leishmaniasis: analysis of the correlation between innate immunity cytokine profile and disease outcome. Scand J Immunol 62:487–495
Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052
Ribeiro PAF, Dias DS, Lage DP, Mendonça DVC, Vale DL, Ramos FF, Carvalho LM, Carvalho AMRS, Steiner BT, Roque MC, Oliveira-da-Silva JA, Oliveira JS, Tavares GSV, Martins VT, Chávez-Fumagalli MA, Roatt BM, Moreira RLF, Menezes-Souza D, Duarte MC et al (2020) Evaluation of the protective efficacy of a Leishmania protein associated with distinct adjuvants against visceral leishmaniasis and in vitro immunogenicity in human cells. Parasitol Res 119:2609–2622
Sasidharan S, Saudagar P (2021) Leishmaniasis: where are we and where are we heading? Parasitol Res 120:1541–1554
Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Müller W, Roers A, Moll H (2013) T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog 9:e1003476
Sharma R, Palanisamy A, Dhama K, Mal G, Singh B, Singh KP (2020) Exploring the possible use of saponin adjuvants in COVID-19 vaccine. Hum Vaccin Immunother 16(12):2944–2953. https://doi.org/10.1080/21645515.2020.1833579
Silva TF, Tomiotto-Pellissier F, Pasquali AKS, Pinto-Ferreira F, Pavanelli WR, Conchon-Costa I, Navarro IT, Caldart ET (2021) Phenotypical and genotypical differences among Leishmania (Leishmania) amazonensis isolates that caused different clinical frames in humans and dogs: a systematic review. Acta Trop 221:106018
Singh OP, Stober CB, Singh AK, Blackwell JM, Sundar S (2012) Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis. PLoS Negl Trop Dis 6:e1874
Sundar S, Singh OP (2018) Molecular diagnosis of visceral leishmaniasis. Mol Diagn Ther 22:443–457
Thakur A, Kaur H, Kaur S (2015) Studies on the protective efficacy of freeze thawed promastigote antigen of Leishmania donovani along with various adjuvants against visceral leishmaniasis infection in mice. Immunobiology 220:1031–1038
Turnis ME, Sawant DV, Szymczak-Workman AL, Andrews LP, Delgoffe GM, Yano H, Beres AJ, Vogel P, Workman CJ, Vignali DAA (2016) Interleukin-35 limits anti-tumor immunity. Immunity 44:316–329
Vale DL, Dias DS, Machado AS, Ribeiro PAF, Lage DP, Costa LE, Steiner BT, Tavares GSV, Ramos FF, Martínez-Rodrigo A, Chávez-Fumagalli MA, Caligiorne RB, de Magalhães-Soares DF, Silveira JAG, Machado-de-Ávila RA, Teixeira AL, Coelho EAF (2019) Diagnostic evaluation of the amastin protein from Leishmania infantum in canine and human visceral leishmaniasis and immunogenicity in human cells derived from patients and healthy controls. Diagn Microbiol Infect Dis 95:134–143
Valentim DL, Ostolin TP, Gusmão MR, Mathias FAS, Cardoso JMO, Roatt BM, Aguiar-Soares RDO, Ruiz JC, Resende DM, Brito RCF, Reis AB (2022) A specific Leishmania infantum polyepitope vaccine triggers Th1-type immune response and protects against experimental visceral leishmaniasis. Cell Immunol 380:104592
Vignali DA, Kuchroo VK (2012) IL-12 family cytokines: immunological playmakers. Nat Immunol 13:722–728
Volpedo G, Huston RH, Holcomb EA, Pacheco-Fernandez T, Gannavaram S, Bhattacharya P, Nakhasi HL, Satoskar AR (2021) From infection to vaccination: reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev Vaccines 20:1431–1446
Wijesooriya H, Samaranayake N, Karunaweera ND (2023) Cytokine and phenotypic cell profiles in human cutaneous leishmaniasis caused by Leishmania donovani. PLoS One 18:e0270722
World Health Organisation (2023). http://www.who.int/topics/leishmaniasis/en/ Available online: Accessed 30 Mar 2023
Xiang XG, Xie Q (2015) IL-35: a potential therapeutic target for controlling hepatitis B virus infection. J Dig Dis 16:1–6
Zorgi NE, Arruda LV, Paladine I, Roque GAS, Araújo TF, Brocchi M, Barral M, Sanchiz Á, Requena JM, Abánades DR, Giorgio S (2020) Leishmania infantum transfected with toxic plasmid induces protection in mice infected with wild type L. infantum or L. amazonensis. Mol Immunol 127:95–106
Funding
The study was supported by grant APQ-02167-21 from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Brazil. The authors also thank the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), FAPEMIG, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for student scholarships.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: EAFC, VTM, MC, BMR, ALT, DUG. Performed the experiments: MMJ, DPL, DLV, CSF, BLP, GJLM, FFR, IAGP, RSB, FL, GSVT, MCD, DMS, BMR. Analyzed the data: EAFC, MACF, VTM, ASG. Wrote the manuscript: EAFC, MACF, MC. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Committee on the Ethical Handling of Research Animals of UFMG approved the study with protocol number 144/2020. In addition, the project was approved by the Ethics Committee on Human Research of University, with protocol number CAAE–32343114.9.0000.5149. A consent form was explained to and signed by all the participants, which accepted to participate and consented to publish their laboratorial results.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Bradford McGwire
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

ESM 1
Supplementary Fig. 1. Representative plots to evaluate the cytoplasmatic cytokine production by flow cytometry. Representative plots of the gating strategy in this study to characterize the IFN-γ, TNF-α, IL-2 and IL-10-producing T-cells frequency are shown by using the Boolean gate strategy (PNG 142 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jesus, M.M., Lage, D.P., Vale, D.L. et al. Immunization with recombinant LiHyp1 protein plus adjuvant is protective against tegumentary leishmaniasis. Parasitol Res 122, 2917–2931 (2023). https://doi.org/10.1007/s00436-023-07981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07981-6